Bergamo® II Series Multiphoton Microscopes

Please Wait
Bergamo® II Series Multiphoton Microscopy Platform
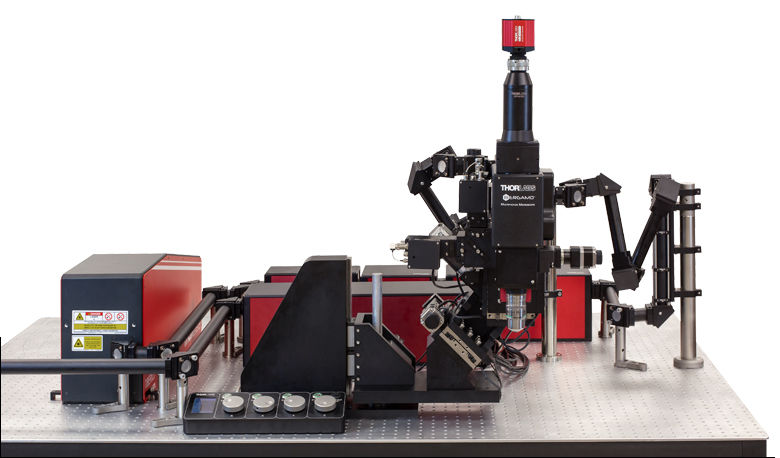
Following the principle that the microscope should conform to the specimen, rather than the other way around, we created a completely modular multiphoton imaging platform that adapts to a wide range of experimental requirements. Our multiphoton imaging systems enable simultaneous readout and manipulation of neuron populations at higher speed, greater depth, and higher intensity than with traditional research techniques, such as using microelectrodes. As shown in the Options at a Glance section below and in the Five-Axis Movement of Rotating Bergamo Microscopes video to the lower right, we have developed a rotating body, which allows the microscope to rotate around the sample. We also offer a selection of rigid bodies, which feature an industry-leading 7.74" throat depth for a large three-dimensional working volume around the objective.
The features of Bergamo II listed in the Highlights, Features, and Modules tabs reflect our focus on developing cutting-edge capabilities without compromising usability. Detailed summaries of all the recommended applications for the Bergamo microscope can be found in the Applications tab. To get users started on designing their own microscope, we provide the Configurations tab, which lists a number of example configurations with key features and suggested applications. This platform can be easily modified or upgraded as experimental needs evolve; see the available add-ons and upgrades in the Retrofits tab.
Bergamo imaging systems have been used to capture impressive sample images (see the Image Gallery tab) and have been featured in numerous publications (see the Publications tab). As shown in the Innovation through Collaboration video to the lower right, the Thorlabs Life Sciences division partnered with Dr. Michael Hausser's research group at the University College London to design a custom Bergamo microscope system to suit their needs. If you have additional questions about our Bergamo imaging systems, please click on the Contact Me button to the right to send us an email.
Five-Axis Movement of Rotating Bergamo Microscopes |
Highlights
- Rapid Volumetric Imaging Using Bessel Beams
- Three-Photon Imaging
- Spatial Light Modulator for Simultaneous Multi-Site Activation
Rapid Volumetric Imaging Using Bessel Beams
In partnership with the Howard Hughes Medical Institute and Prof. Na Ji (University of California at Berkeley), Thorlabs offers a Bessel beam module for our Bergamo® multiphoton laser scanning microscope. In vivo volume imaging of neuronal activity requires both submicron spatial resolution and millisecond temporal resolution. While conventional methods create 3D images by serially scanning a diffraction-limited Gaussian beam, Bessel-beam-based multiphoton imaging relies on an axially elongated focus to capture volumetric images. The excitation beam’s extended depth of field creates a 2D projection of a 3D volume, effectively converting the 2D frame rate into a 3D volumetric rate.
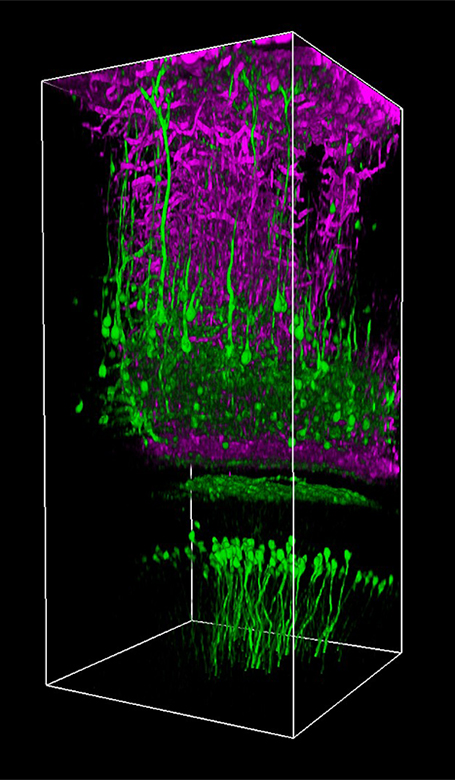
As demonstrated in Ji’s pioneering work, this rapid Bessel beam-based imaging technique has synaptic resolution, capturing Ca2+ dynamics and tuning properties of dendritic spines in mouse and ferret visual cortices. The power of this Bessel-beam-based multiphoton imaging technique is illustrated in the images below, which compare a 300 x 300 μm scan of a Thy1-GFP-M mouse brain slice imaged with Bessel (left) and Gaussian (right) scanning. 45 optical slices taken with a Gaussian focus are vertically stacked to generate a volume image, while the same structural features are visible in a single Bessel scan taken with a 45 μm-long focus. This indicates a substantial gain in volume-imaging speed, making this technique suitable for investigating sparsely labeled samples in-vivo.
If you are interested in upgrading your Bergamo microscope to include the Bessel beam imaging modality, please fill out our multiphoton microscope contact form or call (703) 651-1700. For a list of all the upgrades and add-ons available, please see the Retrofits tab.
Source: Lu R, Sun W, Liang Y, Kerlin A, Bierfeld J, Seelig JD, Wilson DE, Scholl B, Mohar B, Tanimoto M, Koyama M, Fitzpatrick D, Orger MB, and Ji N. "Video-rate volumetric functional imaging of the brain at synaptic resolution." Nature Neuroscience. 2017 Feb 27; 20: 620-628.
A single Bessel scan (left) captures the same structural information obtained from a Gaussian volume scan created by stacking 45 optical sections (right), reducing the total scan time by a factor of 45. The images show a brain slice scanned over a 300 μm x 300 μm area. Scan depth for the Gaussian stack is indicated by the scale bar. Sample Courtesy of Qinrong Zhang, PhD and Matthew Jacobs; the Ji Lab, Department of Physics, University of California, Berkeley.
Click to Enlarge
A Thy1-YFP male mouse, 21 weeks old, imaged at 1300 nm, 326 kHz repetition rate, pulse width ~60 fs. At the top of the cortex (0 µm, 1.1 mW laser power), the window was centered at 2.5 mm lateral and 2 mm posterior from the Bregma point over somatosensory cortex. Courtesy of the Chris Xu Group, Cornell University.
Three-Photon Imaging
For our Bergamo multiphoton microscope, we have developed scan path optics for the 800 - 1800 nm range to open the door to three-photon techniques. Three-photon excitation is ideal for deep tissue imaging and requires a high-pulse-energy excitation source, typically around 1300 nm or 1700 nm. Compared to two-photon imaging, three-photon imaging offers less tissue scattering and reduced out-of-focus background, which results in an improved signal-to-background ratio.
Configurations capable of three-photon imaging, such as the one shown below, can include a dichroic mirror to support simultaneous two-photon and three-photon imaging, as well as electronics to support low-repetition-rate lasers with high bandwidth sampling. ThorImage®LS software has been enhanced with important features for three-photon detection. For instance, users can synchronize the three-photon signal detection to the excitation pulses and control the phase delay for peak signal-to-noise ratio. For more details, please see the ThorImageLS tab.
If you are interested in upgrading your Bergamo microscope to include the three-photon imaging modality, please fill out our multiphoton microscope contact form or call (703) 651-1700. For a list of all the upgrades and add-ons available, please see the Retrofits tab.
Source: Wang T and Xu C. "Three-photon neuronal imaging in deep mouse brain." Optica. 2020; 7 (8): 947-960.
Click to Enlarge
Two- and Three-Photon Imaging Configuration. For more details, please see the Configurations tab.
Spatial Light Modulator for Simultaneous Multi-Site Activation
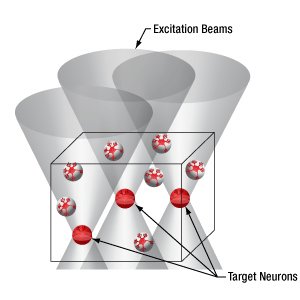
Thorlabs’ Spatial Light Modulator (SLM) uses holography patterns to enable photoactivation of multiple locations in a specimen simultaneously. Designed for two-photon excitation with femtosecond pulses, the SLM manipulates the phase across the stimulation laser beam profile to generate hundreds of user-determined focal points.
The diagrams below illustrate the benefits of using the SLM with two-photon activation over two-photon activation alone and single-photon activation. With single-photon activation, unintended nearby cells as well as the target cell become activated because this technique lacks the ability to target a single cell. This problem can be solved with two-photon activation, which allows single-cell resolution targeting; however, only one cell can be targeted at a time. Two-photon activation with SLM overcomes these limitations by generating a number of focal points and allowing multiple target cells to be activated simultaneously. Each beam can be shaped to improve the efficacy of photoactivation, a crucial feature for activating neural populations at varying depths within a single FOV. The SLM phase mask pattern can be rapidly switched, enabling multiple individual focal points to be targeted independently in any sequence. The calibration process, hologram generation, and external hardware synchronization are entirely managed through the ThorImage®LS software, enabling seamless control. For more details, please see the ThorImageLS tab.
If you are interested in upgrading your Bergamo microscope to include the SLM imaging modality, please fill out our multiphoton microscope contact form or call (703) 651-1700. For a list of all the upgrades and add-ons available, please see the Retrofits tab.
Two-Photon Activation + Spatial Light Modulator
- Generate Multiple Focal Points
- Simultaneously Activate Multiple Target Cells
Click to Enlarge
Single-Photon Activation
- Lack of Depth Control for 3D Neural Activation
- Activate Target Cell and Unintended Nearby Cells
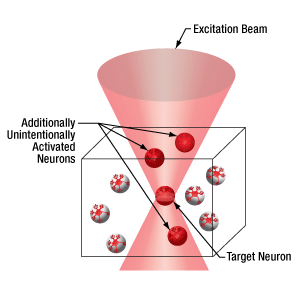
Click to Enlarge
Two-photon activation with SLM (right) allows simultaneous excitation of multiple target cells, which is not possible with single-photon (left) or two-photon (middle) activation.
Features
Many of these features can also be added to existing Thorlabs microscope systems. For more information, please see the Retrofits tab.
| Laser Scanning, Widefield Imaging, and Transmitted Light Imaging | ||
|---|---|---|
| Scan Paths | Resonant-Galvo-Galvo |
|
| Galvo-Resonant |
|
|
| Galvo-Galvo |
|
|
| Spatial Light Modulator |
|
|
| Widefield Viewing |
|
|
| Epi-Illumination | ||
| Dodt Gradient Contrast and DIC Modules |
|
|
| Optical Performance | ||
|---|---|---|
| PMT Configuration |
|
|
| Minimal Distance Between Objective and First Collecting Lens |
|
|
| Periscopes |
|
|
| Scan Paths Designed In-House |
|
|
| Day-to-Day Usage | ||
|---|---|---|
| Several Software Packages |
|
|
| Touchscreen Controller |
|
|
| Easy-to-Reach Emission Filters and Dichroic Holders |
|
|
| Input and Output Triggers |
|
|
| Large User-Adjustable Volume Underneath Objective |
|
|
| ≥90° Rotation of the Focal Plane (Rotating Bodies Only) |
|
|
| Beam Conditioning Modules | ||
|---|---|---|
| Fast Laser Power Modulators |
|
|
| Pockels Cells |
|
|
| Variable Attenuator |
|
|
| Beam Stabilizer |
|
|
| Volume Imaging Using Bessel Beams |
|
|
| Sample Holders | ||
|---|---|---|
| Rigid Stands for Slides, Recording Chambers, or Platforms |
|
|
| XY Platforms Ideal for Micromanipulators |
|
|
| Thorlabs Support | ||
|---|---|---|
| Fully Designed and Manufactured In-House |
|
|
| Modular System Construction |
|
|
| Professional Installation |
|
|
| Quick Support |
|
|
Thorlabs recognizes that each imaging application has unique requirements.
If you have any feedback, questions, or need a quotation, please use our
multiphoton microscopy contact form or call (703) 651-1700.
Bergamo® II Modules
Thorlabs Bergamo® II microscopes are modular systems that can be customized in the design process to meet the exact needs of the experiment. The modules listed below are displayed in a variety of pre-built examples found on our Configurations tab to help provide a starting point for your design.
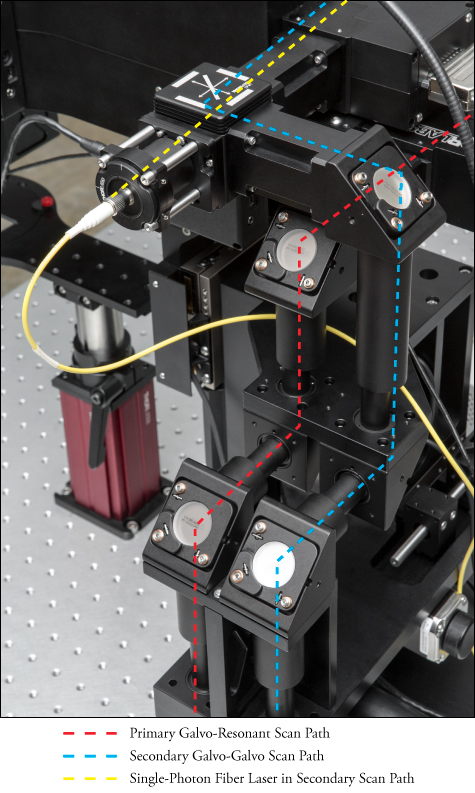
Galvo-Resonant Scanners, Galvo-Galvo Scanners, and Spatial Light Modulators
Bergamo® II microscopes can be configured with one or two co-registered scan paths to propagate, condition, and direct an input laser beam. Each path can utilize a resonant-galvo-galvo scanner, galvo-resonant scanner, galvo-galvo scanner, and/or a spatial light modulator (SLM). These choices allow the user to optimize each experiment as needed for high frame rates, high sensitivity, and/or targeted exposure of the regions of interest.
Resonant-Galvo-Galvo Scanners for Multimodal Scanning
Thorlabs offers 8 kHz and 12 kHz resonant-galvo-galvo (RGG) scanners. The design of our RGG scanners is registered under US Patent 10,722,977. This multimodal scanner provides features of both the galvo-resonant and galvo-galvo scanners in a single scan head. Our 8 kHz scanners utilize the entire field of view and offer a maximum frame rate of 400 fps, while our 12 kHz scanners provide an increased frame rate of 600 fps.
Galvo-Resonant Scanners for High-Speed Imaging
Thorlabs offers 8 kHz and 12 kHz galvo-resonant scanners. Our 8 kHz scanners utilize the entire field of view and offer a maximum frame rate of 400 fps, while our 12 kHz scanners provide an increased frame rate of 600 fps.
Galvo-Galvo Scanners for User-Defined ROI Shapes
Galvo-galvo scanners support user-drawn scan geometries (lines, polylines, squares, and rectangles) and also support custom photoactivation patterns (circles, ellipses, polygons, and points). They offer consistent pixel dwell times for better signal integration and image uniformity.
Spatial Light Modulator for Simultaneous Targeting
Unlike scanners, which physically move from point to point, spatial light modulators (SLMs) use holography to diffract the beam and shape it in a user-defined pattern. This includes general beam shaping as well as the creation of multiple focal points at the FOV; the latter allows multiple sites in a sample to be photoexcited simultaneously.
Click to Enlarge
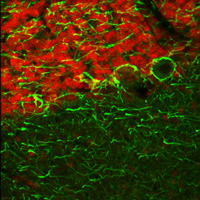
Figure 2. Fast Switching between the optimal excitation wavelengths of 750 nm and 835 nm provides the high contrast seen in this composite image. The two-channel set was collected at an imaging rate of 7 fps.
Click to Enlarge
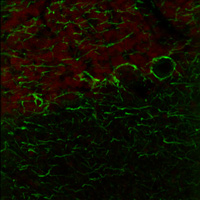
Figure 1. The above image was acquired using single-wavelength excitation at 788 nm, while the optimum excitation wavelengths for the two tags are 750 nm and 850 nm.
Fast Switching Using a
Tunable Femtosecond Laser
With an industry-leading tuning speed of up to 4000 nm/s and a wide 720 to 1060 nm tuning range, the Tiberius® Ti:Sapphire Femtosecond Laser is ideal for fast sequential imaging in multiphoton microscopy applications.
A 25 µm thick sagittal section of an adult rat brain is shown in the images and video to the right. The red channel corresponds to fluorescence from chick anti-neurofilament that is optimally excited at 835 nm, while the green channel corresponds to fluorescence from mouse anti-GFAP that is optimally excited at 750 nm.
Figure 1 shows fluorescence from single-wavelength excitation at 788 nm, which sub-optimally excites the two tags simultaneously. Figure 2 is a composite image of the fluorescence produced by a two-color excitation image sequence acquired at 7 fps where the excitation wavelength was rapidly tuned between 750 and 835 nm. The video in Figure 3 shows the fast-switching used to create the composite image in Figure 2 at 1/16th of the actual speed. When compared to single-wavelength excitation at 788 nm with the same intensity, fast switching offers much higher image contrast as it provides optimal excitation of both fluorophores.
This immunofluorescence sample was prepared by Lynne Holtzclaw of the NICHD Microscopy and Imaging Core Facility, a part of the National Institutes of Health (NIH) in Bethesda, MD.
Click to Enlarge
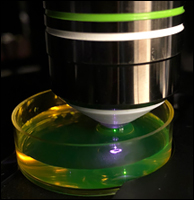
Figure 2. Close-Up of a Gaussian Beam
Click to Enlarge
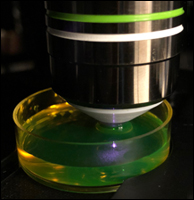
Figure 1. Close-Up of a Bessel Beam
Volumetric Imaging Technique Using Bessel Beams
Thorlabs is excited to offer a new ultra-fast imaging technique that uses a Bessel beam to provide video-rate volumetric functional imaging of neuronal pathways and interactions in vivo. These unique beams are non-diffractive and self-healing, which allows them to maintain a tight focus and even reform as they pass through tissue. This technique is offered for Thorlabs' Bergamo II multiphoton microscopes and Thorlabs' Multiphoton Mesoscope.
The images to the right depict a Bessel beam and a Gaussian beam, respectively. As you can see in the images, the Gaussian beam has a singular point of focus that progressively becomes weaker as it diverges from the central point, whereas the Bessel beam has a beam annulus that maintains its focus.
To read the press release about this new technique, click here.
Click for Details
Rotating Bergamo II systems are outfitted with multi-joint articulating periscopes. This periscope's design offers the enhanced flexibility needed to allow the entire scanning system to be tilted with respect to the sample.

Click to Enlarge
Upright Bergamo II systems are equipped with periscopes that permit the microscope's full travel range in X, Y, and Z to be used without compromising the optical performance.
Periscopes
Most lasers used in multiphoton microscopy are delivered by a free-space beam. The Bergamo II's ability to translate the objective around the focal plane in up to four axes (X, Y, Z, and θ) also requires the beam path to translate along the same axes while maintaining alignment. Bergamo II systems overcome this engineering challenge using multi-jointed periscopes.

Click to Enlarge
Emission filters and dichroic cubes are held behind magnetically sealed doors on the front of the PMT detection module.
Super Broadband Scan Optics
Bergamo II microscopes feature proprietary scan optics that are optimized and corrected for excitation wavelengths within the 450 - 1100 nm, 680 - 1300 nm, or 800 - 1800 nm wavelength range, ideal for photostimulation, two-photon imaging, and three-photon imaging, respectively. These broad ranges, extending from the visible well into the near infrared, were chosen to support the latest widely tunable Ti:Sapphire lasers and OPO systems, as well as dual-output lasers such as the Chameleon Discovery.
Our optics take full advantage of the optical designs used in the low-magnification, high-numerical-aperture objectives by filling the back aperture of the objective up to Ø20 mm. This creates an exceptional scan area that lets you find a region of interest more quickly or simply image more cells at once.
Large-Angle Signal Collection Optics
Deriving the most signal from limited photons is the fundamental goal of any detection system. By positioning the PMTs immediately after the objective (a "non-descanned" geometry), light that is scattered by the sample, which therefore appears to originate outside the objective's field of view, still strikes the PMTs and adds to the collected signal. This is a benefit unique to multiphoton microscopy. Collecting beyond the objective's design field of view greatly enhances overall detection efficiency when imaging deep in tissue.
In the epi direction, we offer signal collection anglesa of 8°, 10°, or 14°, while in the transmitted direction, we offer a signal collection anglea of 13°. Our collection modules can optionally be outfitted with mechanical shutters for photoactivation experiments.
Easy-to-Reach Emission Filters and Dichroic Holders
Bergamo II systems are fully compatible with industry-standard fluorescence filter sets that include Ø25 mm fluorescence filters and 25 mm x 36 mm dichroic mirrors. Unlike competing designs, Thorlabs' detector modules have magnetic holders that make it simple and quick to exchange filters for different measurements.
We also offer detection modules for large-area Ø32 mm fluorescence filters and 32 mm x 44 mm dichroics, which support greater collection angles for increased signal.
Detectors in Epi and Transmitted Directions
We employ high-sensitivity GaAsP PMTs in our multiphoton systems, which can offer high quantum efficiency, aiding in imaging weakly fluorescent or highly photosensitive samples. Our PMTs can either be thermoelectrically cooled for improved sensitivity toward weak signals or non-cooled for a smaller package size and greater numerical aperture. Multialkali PMTs are also available.
All Bergamo® II microscopes can be equipped with either two or four detection channels in the epi direction, and/or two detection channels in the forward direction. The user can configure the forward-direction channels to detect the same fluorescent tags as the epi-direction PMTs, raising the microscope's sensitivity toward thin, weakly fluorescent specimens.
A maximum of four channels can be controlled by the software at a given time.
Multi-Axis Controller with Touchscreen
This controller is specifically designed for rotating Bergamo II microscope bodies. It uses knobs to control up to five motorized axes. On rotating systems, a rocker switch changes between fine objective focusing and translation of the elevator base. Each axis can be disabled on an individual basis in order to maintain a location along the desired direction.
The integrated touchscreen lets two spatial locations be saved and retrieved locally. Up to eight spatial locations can be saved on the computer running ThorImage®LS. The touchscreen also reads out the position of every motor.
Objectives
Bergamo II microscopes accept infinity-corrected objectives with M34 x 1.0, M32 x 0.75, M25 x 0.75, or RMS threads. Together, these options encompass the majority of low-magnification, high-NA objectives used in multiphoton microscopy. With a large field number of 20, our scan optics completely utilize the optical designs of these specialized objectives, offering enhanced light-gathering ability compared to competing microscopes using the same objectives.
Rigid Stand Sample Holders
Thorlabs' Rigid Stands are rotatable, lockable, low-profile platforms for mounting slides, recording chambers, our Z-axis piezo stages, and custom experimental apparatuses. Each fixture is supported by a solid Ø1.5" stainless steel post for passive vibrational damping, which is in turn held to the workstation by the red post holder.
A locking collar maintains the height of the platform, allowing it to easily rotate into and out of the optical path, and a quick-release mechanism holds the post in place once the desired position is achieved.

Click to Enlarge
A Zelux sCMOS Camera and a Quantulux sCMOS Camera
Scientific Cameras
Our low-noise sCMOS and CMOS cameras were designed for full compatibility with Thorlabs’ multiphoton microscopy systems. Useful for widefield and fluorescence microscopy, they are capable of visualizing in vitro and in vivo samples using reflected light and fluorescence emission. They work in conjunction with the epi-fluorescence module to help locate fiducial markers, and they also enable imaging modalities that do not require laser exposure.
Thorlabs' cameras are driven by our internally developed ThorCam software package. The sCMOS camera is available with a 2.1 MP sensor, and our CMOS cameras are available with either a 1.3 MP, 2.3 MP, 5 MP, 8.9 MP, or 12.3 MP sensor. Generally speaking, cameras with lower resolution offer higher maximum frame rates. These cameras also feature a separate auxiliary port that permits the image acquisition to be driven by an external electrical trigger signal.
Bergamo® II microscopes are also directly compatible with any camera using industry-standard C-mount or CS-mount threads.

Click to Enlarge
Trans-Illumination Module, Motorized Condenser Stage, and
Rigid Stand Sample Holder Underneath the Objective
User-Installable Dodt Contrast and DIC Imaging Modules
The modular construction of the Bergamo® II makes it exceptionally easy for the user to convert the microscope between in vitro and in vivo applications. Our user-installable trans-illumination modules for Dodt contrast, laser-scanned Dodt contrast, and differential interference contrast (DIC) take less than 5 minutes to attach or remove from the microscope body. These modules are available for both rotating and upright bodies.
Each option is paired with our basic 3-axis controller, which optimizes the illumination conditions by translating our motorized condenser stage over a 1" range. This versatile design is compatible with air and high-NA oil immersion condensers designed by Nikon.
To complement these modules, we manufacture slim-profile rigid stand sample holders that are ideal for positioning slides between the transmitted light module and the objective.
Thorlabs recognizes that each imaging application has unique requirements.
If you have any feedback, questions, or need a quotation, please use our
multiphoton microscopy contact form or call (703) 651-1700.
Thorlabs' Bergamo® II Series Multiphoton Microscopy Platform is a powerful tool adapted to meet experimental needs across a wide range of research fields. Click on the images below to explore how a Bergamo can and has been utilized for each application.
Example Configurations
Explore the details of example Bergamo II rotating, XYZ, and Z-axis system configurations by clicking on the expandable sections below. The modular nature of our multiphoton microscopy platform allows us to modify configurations to meet individual experimental needs or adjust the functionality of a microscope after installation; more information on modules featured in the systems below can be found on the Modules tab.
| Configuration | System Highlights |
||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rotating Body | |||||||||||||||||||||||||||||||||||||||||||||
| Configuration B243: Multi-Target Photoactivation |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for Multi-Target Photoactivation Configuration
Our spatial light modulator (SLM) manipulates the phase of the stimulation laser beam to generate hundreds of user-determined focal points. The SLM phase mask pattern can be rapidly switched, enabling multiple individual focal points to be targeted independently of each other. Each beamlet can be shaped to improve the efficacy of photoactivation; a crucial feature for activating neural populations at varying depths within a single FOV. This configuration utilizes two separate lasers for imaging and photostimulation; in addition to the highly configurable nature of the Bergamo microscope system, the scanning paths can be adjusted for a dual-output laser source.  Click to Enlarge The SLM is installed on the secondary scan path, enabling photostimulation simultaneous with multiphoton imaging.  Click to Enlarge The Tiberius fs tunable laser is used for multiphoton imaging, while Menlo Systems' BlueCut fiber laser is used for photostimulation. |
|||||||||||||||||||||||||||||||||||||||||||||
| Configuration B242: Two- and Three-Photon Imaging |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for 2P / 3P Imaging Configuration
This dual-path configuration uses both galvo-resonant and galvo-galvo scanners and infrared wavelength scanning optics to image second- and third-harmonic generation (SHG and THG).  Click to Enlarge The primary and secondary scan path optics are optimized for 2P and 3P imaging, respectively. The beam paths are co-registered for simultaneous multi-channel imaging.  Click to Enlarge View your subject at angles up to +90° with our Rotating Microscope Bases. The body rotation is centered on the focal point of your objective, allowing free movement without the need to refocus. |
|||||||||||||||||||||||||||||||||||||||||||||
| Configuration B251: Dual-Modality and In Vivo Imaging |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for Dual-Modality and In Vivo Imaging ConfigurationThis dual-modality and in vivo imaging configuration uses a resonant-galvo-galvo scanner to take high-resolution images. This scanner provides all the speed of a resonant-galvo scanner while enabling user-defined regions to be chosen.
 Click to Enlarge The microscope can be placed within a soundbox for sensitive auditory studies, as shown here. |
|||||||||||||||||||||||||||||||||||||||||||||
| Configuration B241: In Vivo Two-Photon Imaging |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for In Vivo 2P Imaging ConfigurationThis rotating Bergamo microscope configuration is compact enough to be placed in an enclosure, enabling sound or light-sensitive in vivo studies to be performed.
 Click to Enlarge 2P Rotating Microscope Encased Inside a Soundbox |
|||||||||||||||||||||||||||||||||||||||||||||
| Upright XYZ Body | |||||||||||||||||||||||||||||||||||||||||||||
| Configuration B252: Bessel Beam |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for Bessel Beam Configuration
Fully equipped for Bessel beam microscopy, this configuration features both a resonant-galvo-galvo scanner on the primary path and a galvo-galvo scanner on the secondary path. The system uses a dual-output laser for multiphoton imaging.  Click for Details The large breadboard system shown above allows both samples and supplementary equipment to be aligned in the focal plane of the objective. Here, a motorized micromanipulator is mounted onto the breadboard to manipulate cells for patch-clamp recordings.  Click to Enlarge A custom enclosure can be added to house beam conditioning modules and other components, creating a light-tight region in which parts may be altered without affecting the rest of the beam path.  Click to Enlarge The resonant-galvo-galvo scanner on the primary path allows for multimodal scanning, while the galvo-galvo scanner on the secondary path can be used for simultaneous photoactivation. |
|||||||||||||||||||||||||||||||||||||||||||||
| Configuration B262: Dual-Path with Confocal Imaging |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for Dual-Path Confocal Configuration
This multi-path, multi-modality configuration allows for a range of experimental conditions to be observed using any combination of multiphoton, confocal, and epi-fluorescence imaging, along with photoactivation. Multiphoton imaging with photoactivation is enabled by two beam paths; the primary with a galvo-resonant scanner for high-speed imaging, and the secondary with a galvo-galvo scanner for area-specific photoactivation. The four-channel confocal laser connects through a fiber bulkhead to the secondary scan path, and the four-channel PMT detection module uses an additional port at the back focal plane of the objective. Epi-fluorescence is enabled by a six-filter-turret epi-illuminator module and sCMOS Quantalux camera. 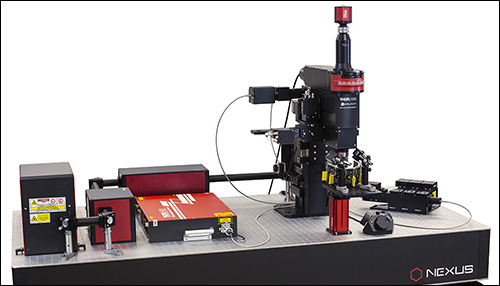 Click to Enlarge Thorlabs provides a variety of sample mounting options. The breadboard insert on the rigid stand provides the space and stability for a VR fly theater and supplementary cameras to be mounted below the objective, as shown. |
|||||||||||||||||||||||||||||||||||||||||||||
| Configuration B231: Simple XYZ Imaging |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for Simple XYZ Configuration
This configuration is well-balanced for both in vitro and fixed stage in vivo microscopy research. The modular system with a removable trans-illumination module provides high versatility for multiple experimental techniques, imaging modalities, and sample subjects.  Click to Enlarge The ample space under the microscope enables large setups with supplemental equipment to be easily arranged under the objective. Here, we show a Drosophila VR theater stage along with two additional cameras. The setup can be synchronized with image acquisition using ThorSync, an add-on to our ThorImageLS software.  Click to Enlarge Any one of our PMTs can be replaced with a free-space photodetector for signal detection of other imaging modalities. This image shows a variation of the microscope where one PMT is replaced with the DET10A2 for reflected signal detection. |
|||||||||||||||||||||||||||||||||||||||||||||
| Upright Z-Axis Body | |||||||||||||||||||||||||||||||||||||||||||||
| Configuration B211: Video and High-Speed Imaging |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for Video Imaging Configuration
This configuration enables video-rate, sequential two-photon imaging to study fast dynamic biological and chemical processes.  Click to Enlarge Rigid stands provide stability for numerous sample mounting options, including the multi-camera VR theater shown here for Drosophila studies.  Click to Enlarge Galvo-resonant scanners are available at 8 kHz and 12 kHz resonant frequencies. |
|||||||||||||||||||||||||||||||||||||||||||||
| Configuration B201: Simple Z-Axis Imaging |
Key Features | Suggested Applications | |||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||
Additional Specifications for Simple Z-Axis Imaging ConfigurationThis configuration provides the simplest microscopy system for studies that require multiphoton imaging at the sample plane without unnecessary features. Please note that this configuration requires a power attenuator (not pictured).
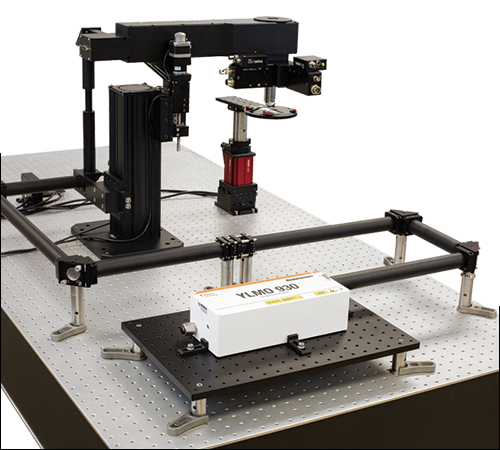 Click to Enlarge Menlo Systems' YLMO 930 nm laser is shown here as the primary laser source. Imaging optics may be tuned for short or long-wavelength multiphoton imaging, and the galvo-galvo scanner switched to accommodate a larger or smaller beam diameter. |
|||||||||||||||||||||||||||||||||||||||||||||
Thorlabs recognizes that each imaging application has unique requirements.
If you have any feedback, questions, or need a quotation, please use our
multiphoton microscopy contact form or call (703) 651-1700.

Click to Enlarge
Trans-Illumination Add-On for Rotating Bergamo II Systems
Retrofit Options for Existing Multiphoton Systems
- Upgrades to the Functionality of Existing Microscope Infrastructure and Components
- Add-On Components to Increase Capabilities
Thorlabs' modular design enables our microscopes to continually evolve with experimental needs. Customers with previous model microscopes and product lines for multiphoton microscopy have the flexibility to update their infrustructure or add to their existing components as their microscopy needs change. See the expandable tables below for options available for each of our multiphoton system lines, and contact us for additional information about incorporating an upgrade or add-on into your system.
Please note that certain upgrades and add-ons require an on-site visit by one of our specialists for installation.
| Bergamo II Compatible Upgrades and Add-Ons |
|---|
| Bergamo I (B-Scope) Compatible Upgrades and Add-Ons |
|---|
| Acerra (A-Scope) Compatible Upgrades and Add-Ons |
|---|
Images Taken Using Bergamo® Systems
Selected Publications Using Thorlabs' Imaging Systems
2023
Tsuji, M., Nishizuka, Y. & Emoto, K. (2023). Threat gates visual aversion via theta activity in Tachykinergic neurons. Nature Communications, 14(1), 3987.
Villanueva, C. B., Stephensen, H. J. T., Mokso, R., Benraiss, A., Sporring, J., Goldman, S.A. (2023). Astrocytic engagement of the corticostriatal synaptic cleft is disrupted in a mouse model of Huntington's disease. Proceedings of the National Academy of Sciences, 120(24), e2210719120.
Holstein-Rønsbo, S., Gan, Y., Giannetto, M.J. et al. (2023). Glymphatic influx and clearance are accelerated by neurovascular coupling. Nature Neuroscience, 26, 1042–1053.
Serradj, N., Marino, F., Moreno-López, Y. et al. (2023). Task-specific modulation of corticospinal neuron activity during motor learning in mice. Nature Communications, 14(1), 2708.
Inayat, S., McAllister, B. B., Whishaw, I. Q., & Mohajerani, M. H. (2023). Hippocampal conjunctive and complementary CA1 populations relate sensory events to movement. Iscience, 26(4), 106481.
Untiet, V., Beinlich, F. R., Kusk, P., Kang, N., Ladrón-de-Guevara, A., Song, W., ... & Nedergaard, M. (2023). Astrocytic chloride is brain state dependent and modulates inhibitory neurotransmission in mice. Nature Communications, 14(1), 1871.
Anthoney, N., Tainton-Heap, L., Luong, H., Notaras, E., Zhao, Q., Perry, T., ... & van Swinderen, B. (2023). Experimentally induced active and quiet sleep engage non-overlapping transcriptomes in Drosophila. bioRxiv, 535331.
Boster, K. A., Cai, S., Ladrón-de-Guevara, A., Sun, J., Zheng, X., Du, T., ... & Kelley, D. H. (2023). Artificial intelligence velocimetry reveals in vivo flow rates, pressure gradients, and shear stresses in murine perivascular flows. Proceedings of the National Academy of Sciences, 120(14), e2217744120.
Scarpetta, V., Bodaleo, F., Salio, C., Agarwal, A., Sassoè-Pognetto, M., & Patrizi, A. (2023). Morphological and mitochondrial changes in murine choroid plexus epithelial cells during healthy aging. Fluids and Barriers of the CNS, 20(1), 1-16.
McDermott, K. D., Frechou, M. A., Jordan, J. T., Martin, S. S., & Gonçalves, J. T. (2023). Delayed formation of neural representations of space in aged mice. bioRxiv, 531021.
Matthews, M. D., Cook, E., Naguib, ,N., Wiesner, U. B., & Lewis, K. J. (2023). Intravital imaging of osteocyte integrin dynamics with locally injectable fluorescent nanoparticles. Bone, 174, 116830.
Tort-Colet, N., Resta, F., Montagni, E., Pavone, F., Allegra Mascaro, A. L., & Destexhe, A. (2023). Assessing brain state and anesthesia level with two-photon calcium signals. Scientific Reports, 13(1), 3183.
Esteves, I. M., Chang, H., Neumann, A. R., & McNaughton, B. L. (2023). Consolidation of cellular memory representations in superficial neocortex. Iscience, 26(2), 105970.
Quezada, A., Ward, C., Bader, E. R., Zolotavin, P., Altun, E., Hong, S., ... & Hébert, J. M. (2023). An in vivo platform for rebuilding functional neocortical tissue. Bioengineering, 10(2), 263.
Yang, J. Y., O’Connell, T. F., Hsu, W. M. M., Bauer, M. S., Dylla, K. V., Sharpee, T. O., & Hong, E. J. (2023). Restructuring of olfactory representations in the fly brain around odor relationships in natural sources. bioRxiv, 528627.
Carlton, A. J., Jeng, J. Y., Grandi, F. C., De Faveri, F., Ceriani, F., De Tomasi, L., ... & Marcotti, W. (2023). A critical period of prehearing spontaneous Ca2+ spiking is required for hair-bundle maintenance in inner hair cells. The EMBO Journal, 42(4), e112118.
Hamling, K. R., Zhu, Y., Auer, F., & Schoppik, D. (2023). Tilt In Place Microscopy (TIPM): a simple, low-cost solution to image neural responses to body rotations. Journal of Neuroscience, 43(6), 936-948.
Vestergaard, M., Carta, M., Güney, G., & Poulet, J. F. A. (2023). The cellular coding of temperature in the mammalian cortex. Nature, 614(7949), 725-731.
Contreras-López, R., Alatriste-León, H., Díaz-Hernández, E., Ramírez-Jarquín, J. O., & Tecuapetla, F. (2023). The deep cerebellar nuclei to striatum disynaptic connection contributes to skilled forelimb movement. Cell Reports, 42(1), 112000.
Mabuchi, Y., Cui, X., Xie, L., Kim, H., Jiang, T., & Yapici, N. (2023). GABA-mediated inhibition in visual feedback neurons fine-tunes Drosophila male courtship. bioRxiv, 525544.
Das, A., Margevicius, D., Borovicka, J., Icardi, J., Patel, D., Paquet, M. E., & Dana, H. (2023). Enhanced detection sensitivity of neuronal activity patterns using CaMPARI1 vs. CaMPARI2. Frontiers in Neuroscience, 16, 2291.
Lee, S., Lee, K., Choi, M., & Park, J. (2023). Implantable acousto-optic window for monitoring ultrasound-mediated neuromodulation in vivo. Neurophotonics, 9(3), 032203.
Zhuo, G. Y., Chen, M. C., Lin, T. Y., Lin, S. T., Chen, D. T. L., & Lee, C. W. S. (2023). Opioid-Modulated Receptor Localization and Erk1/2 Phosphorylation in Cells Coexpressing μ-Opioid and Nociceptin Receptors. International Journal of Molecular Sciences, 24(2), 1048.
Diaz-Cuadros, M., Miettinen, T. P., Skinner, O. S., Sheedy, D., Díaz-García, C. M., Gapon, S., ... & Pourquié, O. (2023). Metabolic regulation of species-specific developmental rates. Nature, 613(7944), 550-557.
Evrard, M., Mansuryan, T., Couderc, V., Désévédavy, F., Strutynski, C., Dussauze, M., ... & Smektala, F. (2023). Highly Nonlinear Multimode Tellurite Fibers: From Glass Synthesis to Practical Applications in Multiphoton Imaging. Advanced Photonics Research, 4(1), 2200213.
2022
Graham, R. T., Parrish, R. R., Alberio, L., Johnson, E. L., Owens, L., & Trevelyan, A. J. (2022). Optogenetic stimulation reveals a latent tipping point in cortical networks during ictogenesis. Brain, awac487.
Shiozaki, H. M., Wang, K., Lillvis, J. L., Xu, M., Dickson, B. J., & Stern, D. L. (2022). Neural coding of distinct motor patterns during Drosophila courtship song. bioRxiv, 520499.
Fisher, Y. E., Marquis, M., D’Alessandro, I., & Wilson, R. I. (2022). Dopamine promotes head direction plasticity during orienting movements. Nature, 612(7939), 316-322.
Wang, X., Delle, C., Asiminas, A., Akther, S., Vittani, M., Brxxxgger, P., ... & Hirase, H. (2022). Liver-secreted fluorescent blood plasma markers enable chronic imaging of the microcirculation. Cell Reports Methods, 2(10), 100302.
Lu, Y., Wei, X., Li, W., Wu, X., Chen, C., Li, G., ... & Gan, W. B. (2022). Large-volume and deep brain imaging in rabbits and monkeys using COMPACT two-photon microscopy. Scientific Reports, 12(1), 17736.
Huang, J. S., Kunkhyen, T., Rangel, A. N., Brechbill, T. R., Gregory, J. D., Winson-Bushby, E. D., ... & Cheetham, C. E. (2022). Immature olfactory sensory neurons provide behaviourally relevant sensory input to the olfactory bulb. Nature Communications, 13(1), 6194.
Govindaraju, I., Muraleedharan, M., Maidin, S., Chakraborty, I., Zhuo, G. Y., Mahato, K. K., & Mazumder, N. (2022). Investigation of the effect of gamma irradiation on the morphological structures of starch using advanced microscopic techniques. In Frontiers in Optics, JTu5A-74.
Hwang, F. J., Roth, R. H., Wu, Y. W., Sun, Y., Kwon, D. K., Liu, Y., & Ding, J. B. (2022). Motor learning selectively strengthens cortical and striatal synapses of motor engram neurons. Neuron, 110(17), 2790-2801.
Yue, Y., Ash, R. T., Boyle, N., Kinter, A., Li, Y., Zeng, C., & Lu, H. (2022). MeCP2 deficiency impairs motor cortical circuit flexibility associated with motor learning. Molecular Brain, 15(1), 1-12.
Liu, Z., Lu, X., Villette, V., Gou, Y., Colbert, K. L., Lai, S., Guan, S., ... & St-Pierre, F. (2022). Sustained deep-tissue voltage recording using a fast indicator evolved for two-photon microscopy. Cell, 185(18), 3408-3425.
Aragon, M. J., Mok, A. T., Shea, J., Wang, M., Kim, H., Barkdull, N., ... & Yapici, N. (2022). Multiphoton imaging of neural structure and activity in Drosophila through the intact cuticle. Elife, 11, e69094.
Matheson, A. M., Lanz, A. J., Medina, A. M., Licata, A. M., Currier, T. A., Syed, M. H., & Nagel, K. I. (2022). A neural circuit for wind-guided olfactory navigation. Nature Communications, 13(1), 4613.
Chornyy, S., Borovicka, J. A., Patel, D., Shin, M. K., Vázquez-Rosa, E., Miller, E., ... & Dana, H. (2022). Longitudinal in vivo monitoring of axonal degeneration after brain injury. Cell Reports Methods, 3(5), 100481.
Sheng, W., Zhao, X., Huang, X., & Yang, Y. (2022). Real-time image processing toolbox for all-optical closed-loop control of neuronal activities. Frontiers in Cellular Neuroscience, 16, 917713.
Tadres, D., Shiozaki, H. M., Tastekin, I., Stern, D. L., & Louis, M. (2022). An essential experimental control for functional connectivity mapping with optogenetics. bioRxiv, 493610.
Koveal, D., Rosen, P. C., Meyer, D. J., Díaz-García, C. M., Wang, Y., Cai, L. H., ... & Yellen, G. (2022). A high-throughput multiparameter screen for accelerated development and optimization of soluble genetically encoded fluorescent biosensors. Nature Communications, 13(1), 2919.
Fukuda, M., Matsumura, T., Suda, T., & Hirase, H. (2022). Depth-targeted intracortical microstroke by two-photon photothrombosis in rodent brain. Neurophotonics, 9(2), 021910-021910.
Jackson, J. G., Krizman, E., Takano, H., Lee, M., Choi, G. H., Putt, M. E., & Robinson, M. B. (2022). Activation of Glutamate Transport Increases Arteriole Diameter in vivo: Implications for Neurovascular Coupling. Frontiers in Cellular Neuroscience, 16, 831061.
Du, T., Mestre, H., Kress, B. T., Liu, G., Sweeney, A. M., Samson, A. J., ... & Nedergaard, M. (2022). Cerebrospinal fluid is a significant fluid source for anoxic cerebral oedema. Brain, 145(2), 787-797.
Govindaraju, I., Zhuo, G. Y., Chakraborty, I., Melanthota, S. K., Mal, S. S., Sarmah, B., ... & Mazumder, N. (2022). Investigation of structural and physico-chemical properties of rice starch with varied amylose content: A combined microscopy, spectroscopy, and thermal study. Food Hydrocolloids, 122, 107093.
Choe, K., Hontani, Y., Wang, T., Hebert, E., Ouzounov, D. G., Lai, K., ... & Xu, C. (2022). Intravital three-photon microscopy allows visualization over the entire depth of mouse lymph nodes. Nature Immunology, 23(2), 330-340.
Spivak, Y. M., & Lemeshko, P. S. (2022). Multiphoton microscopy of mesoporous silicon. In Journal of Physics: Conference Series. 2227(1), 012013.
2021
Maset, A., Albanesi, M., di Soccio, A., Canova, M., Dal Maschio, M., & Lodovichi, C. (2021). Aberrant Patterns of Sensory-Evoked Activity in the Olfactory Bulb of LRRK2 Knockout Mice. Cells, 10(11), 3212.
Schmidt, E. R., Zhao, H. T., Park, J. M., Dipoppa, M., Monsalve-Mercado, M. M., Dahan, J. B., ... & Polleux, F. (2021). A human-specific modifier of cortical connectivity and circuit function. Nature, 599(7886), 640-644.
Hermans, L., Kaynak, M., Braun, J., Lobato Ríos, V., Chen, C. L., Günel, S., ... & Ramdya, P. (2021). Long-term imaging of the ventral nerve cord in behaving adult Drosophila. bioRxiv, 463778.
Carlsen, E. M. M., Falk, S., Skupio, U., Robin, L., Pagano Zottola, A. C., Marsicano, G., & Perrier, J. F. (2021). Spinal astroglial cannabinoid receptors control pathological tremor. Nature Neuroscience, 24(5), 658-666.
Moussa, N. O., Mansuryan, T., Hage, C. H., Fabert, M., Krupa, K., Tonello, A., ... & Couderc, V. (2021). Spatiotemporal beam self-cleaning for high-resolution nonlinear fluorescence imaging with multimode fiber. Scientific Reports, 11(1), 18240.
Eleftheriou, C. G., Corona, C., Khattak, S., Alam, N. M., Ivanova, E., Bianchimano, P., ... & Sagdullaev, B. T. (2021). Retinoschisin deficiency induces persistent aberrant waves of activity affecting neuroglial signaling in the retina. Journal of Neuroscience, 42(36), 6983-7000.
Han, J., Kim, S., Choi, P., Lee, S., Jo, Y., Kim, E., & Choi, M. (2021). Robust functional imaging of taste sensation with a Bessel beam. Biomedical Optics Express, 12(9), 5855-5864.
Favuzzi, E., Huang, S., Saldi, G. A., Binan, L., Ibrahim, L. A., Fernández-Otero, M., ... & Fishell, G. (2021). GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell, 184(15), 4048-4063.
Chornyy, S., Das, A., Borovicka, J. A., Patel, D., Chan, H. H., Hermann, J. K., ... & Dana, H. (2021). Cellular-resolution monitoring of ischemic stroke pathologies in the rat cortex. Biomedical Optics Express, 12(8), 4901-4919.
Malina, K. C. K., Tsivourakis, E., Kushinsky, D., Apelblat, D., Shtiglitz, S., Zohar, E., ... & Spiegel, I. (2021). NDNF interneurons in layer 1 gain-modulate whole cortical columns according to an animal’s behavioral state. Neuron, 109(13), 2150-2164.
Bruzzone, M., Chiarello, E., Albanesi, M., Miletto Petrazzini, M. E., Megighian, A., Lodovichi, C., & Dal Maschio, M. (2021). Whole brain functional recordings at cellular resolution in zebrafish larvae with 3D scanning multiphoton microscopy. Scientific Reports, 11(1), 11048.
Mridha, Z., de Gee, J. W., Shi, Y., Alkashgari, R., Williams, J., Suminski, A., ... & McGinley, M. J. (2021). Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nature Communications, 12(1), 1539.
Ivanova, E., Corona, C., Eleftheriou, C. G., Bianchimano, P., & Sagdullaev, B. T. (2021). Retina-specific targeting of pericytes reveals structural diversity and enables control of capillary blood flow. Journal of Comparative Neurology, 529(6), 1121-1134.
Hung, C. W., Mazumder, N., Lin, D. J., Chen, W. L., Lin, S. T., Chan, M. C., & Zhuo, G. Y. (2021). Label-free characterization of collagen crosslinking in bone-engineered materials using nonlinear optical microscopy. Microscopy and Microanalysis, 27(3), 587-597.
Hontani, Y., Xia, F., & Xu, C. (2021). Multicolor three-photon fluorescence imaging with single-wavelength excitation deep in mouse brain. Science Advances, 7(12), eabf3531.
Resnik, J., & Polley, D. B. (2021). Cochlear neural degeneration disrupts hearing in background noise by increasing auditory cortex internal noise. Neuron, 109(6), 984-996.
Tainton-Heap, L. A., Kirszenblat, L. C., Notaras, E. T., Grabowska, M. J., Jeans, R., Feng, K., ... & van Swinderen, B. (2021). A paradoxical kind of sleep in Drosophila melanogaster. Current Biology, 31(3), 578-590.
Hortholary, T., Carrion, C., Chouzenoux, E., Pesquet, J. C., & Lefort, C. (2021). Multiplex-multiphoton microscopy and computational strategy for biomedical imaging. Microscopy Research and Technique, 84(7), 1553-1562.
Clayton, K. K., Williamson, R. S., Hancock, K. E., Tasaka, G. I., Mizrahi, A., Hackett, T. A., & Polley, D. B. (2021). Auditory corticothalamic neurons are recruited by motor preparatory inputs. Current Biology, 31(2), 310-321.
Esteves, I. M., Chang, H., Neumann, A. R., Sun, J., Mohajerani, M. H., & McNaughton, B. L. (2021). Spatial information encoding across multiple neocortical regions depends on an intact hippocampus. Journal of Neuroscience, 41(2), 307-319.
Schumacher, J. W., McCann, M. K., Maximov, K. J., & Fitzpatrick, D. (2021). Selective enhancement of neural coding in V1 underlies fine-discrimination learning in tree shrew. Current Biology, 32(15), 3245-3260.
2020
Keller, A. J., Dipoppa, M., Roth, M. M., Caudill, M. S., Ingrosso, A., Miller, K. D., & Scanziani, M. (2020). A disinhibitory circuit for contextual modulation in primary visual cortex. Neuron, 108(6), 1181-1193.
Lu, J., Behbahani, A. H., Hamburg, L., Westeinde, E. A., Dawson, P. M., Lyu, C., ... & Wilson, R. I. (2020). Transforming representations of movement from body-to world-centric space. Nature, 601(7891), 98-104.
Scholl, B., Thomas, C. I., Ryan, M. A., Kamasawa, N., & Fitzpatrick, D. (2020). Cortical response selectivity derives from strength in numbers of synapses. Nature, 590(7844), 111-114.
Robinson, N. T., Descamps, L. A., Russell, L. E., Buchholz, M. O., Bicknell, B. A., Antonov, G. K., ... & Häusser, M. (2020). Targeted activation of hippocampal place cells drives memory-guided spatial behavior. Cell, 183(6), 1586-1599.
Fan, J. L., Rivera, J. A., Sun, W., Peterson, J., Haeberle, H., Rubin, S., & Ji, N. (2020). High-speed volumetric two-photon fluorescence imaging of neurovascular dynamics. Nature Communications, 11(1), 6020.
Okubo, T. S., Patella, P., D’Alessandro, I., & Wilson, R. I. (2020). A neural network for wind-guided compass navigation. Neuron, 107(5), 924-940.
Mazzarda, F., D'Elia, A., Massari, R., De Ninno, A., Bertani, F. R., Businaro, L., ... & Mammano, F. (2020). Organ-on-chip model shows that ATP release through connexin hemichannels drives spontaneous Ca 2+ signaling in non-sensory cells of the greater epithelial ridge in the developing cochlea. Lab on a Chip, 20(16), 3011-3023.
Bowen, Z., Winkowski, D. E., & Kanold, P. O. (2020). Functional organization of mouse primary auditory cortex in adult C57BL/6 and F1 (CBAxC57) mice. Scientific Reports, 10(1), 10905.
Feng, K., Sen, R., Minegishi, R., Dübbert, M., Bockemühl, T., Büschges, A., & Dickson, B. J. (2020). Distributed control of motor circuits for backward walking in Drosophila. Nature Communications, 11(1), 6166.
Keller, A. J., Roth, M. M., & Scanziani, M. (2020). Feedback generates a second receptive field in neurons of the visual cortex. Nature, 582(7813), 545-549.
Eschbach, C., Fushiki, A., Winding, M., Afonso, B., Andrade, I. V., Cocanougher, B. T., ... & Zlatic, M. (2020). Circuits for integrating learnt and innate valences in the fly brain. bioRxiv, 058339.
Montgomery, M. K., Kim, S. H., Dovas, A., Zhao, H. T., Goldberg, A. R., Xu, W., ... & Hillman, E. M. (2020). Glioma-induced alterations in neuronal activity and neurovascular coupling during disease progression. Cell Reports, 31(2), 107500.
Shiozaki, H. M., Ohta, K., & Kazama, H. (2020). A multi-regional network encoding heading and steering maneuvers in Drosophila. Neuron, 106(1), 126-141.
Mestre, H., Du, T., Sweeney, A. M., Liu, G., Samson, A. J., Peng, W., ... & Nedergaard, M. (2020). Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science, 367(6483), eaax7171.
Jeng, J. Y., Ceriani, F., Hendry, A., Johnson, S. L., Yen, P., Simmons, D. D., ... & Marcotti, W. (2020). Hair cell maturation is differentially regulated along the tonotopic axis of the mammalian cochlea. The Journal of Physiology, 598(1), 151-170.
Kovacs-Oller, T., Ivanova, E., Bianchimano, P., & Sagdullaev, B. T. (2020). The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discovery, 6(1), 39.
Oe, Y., Wang, X., Patriarchi, T., Konno, A., Ozawa, K., Yahagi, K., ... & Hirase, H. (2020). Distinct temporal integration of noradrenaline signaling by astrocytic second messengers during vigilance. Nature Communications, 11(1), 471.
2019
Romero, S., Hight, A. E., Clayton, K. K., Resnik, J., Williamson, R. S., Hancock, K. E., & Polley, D. B. (2019). Cellular and widefield imaging of sound frequency organization in primary and higher order fields of the mouse auditory cortex. Cerebral Cortex, 30(3), 1603-1622.
Nardin, C., Peres, C., Mazzarda, F., Ziraldo, G., Salvatore, A. M., & Mammano, F. (2019). Photosensitizer activation drives apoptosis by interorganellar Ca2+ transfer and superoxide production in bystander cancer cells. Cells, 8(10), 1175.
Scholl, B., Wilson, D. E., Jaepel, J., & Fitzpatrick, D. (2019). Functional logic of layer 2/3 inhibitory connectivity in the ferret visual cortex. Neuron, 104(3), 451-457.
Ceriani, F., Johnson, S. L., Sedlacek, M., Hendry, A., Kachar, B., Marcotti, W., & Mammano, F. (2019). Dynamic coupling of cochlear inner hair cell intrinsic Ca2+ action potentials to Ca2+ signaling of non-sensory cells. bioRxiv, 731851.
Brawek, B and Garaschuk O. (2019). Single-cell electroporation for measuring in vivo calcium dynamics in microglia. Microglia Methods and Protocols, 231-241.
Brawek, B., Olmedillas del Moral, M., & Garaschuk, O. (2019). In vivo visualization of microglia using tomato lectin. Microglia Methods and Protocols, 165-175.
Liang, Y. & Garaschuk O. (2019). Labeling microglia with genetically encoded calcium indicators. Microglia Methods and Protocols, 243–265.
Royzen, F., Williams, S., Fernandez, F. R., & White, J. A. (2019). Balanced synaptic currents underlie low-frequency oscillations in the subiculum. Hippocampus, 29(12), 1178-1189.
Rathore, A. P. S., Mantri, C. K., Aman, S. A. B., Syenina, A., Ooi, J., Jagaraj, C. J., ... & St. John, A. L. (2019). Dengue virus-elicited tryptase induces endothelial permeability and shock. Journal of Clinical Investiagtion, 129(10), 4180-4193.
Stringer, C., Pachitariu, M., Steinmetz, N., Carandini, M., & Harris, K. D. (2019). High-dimensional geometry of population responses in visual cortex. Nature, 571(7765), 361–365.
Ziraldo, G., Buratto, D., Kuang, Y., Xu, L., Carrer, A., Nardin, C., ... & Mammano, F. (2019). A human-derived monoclonal antibody targeting extracellular connexin domain selectively modulates hemichannel function. Frontiers in Physiology, 10, 392.
Díaz-García, C. M., Lahmann, C., Martínez-François, J. R., Li, B., Koveal, D., Nathwani N., ... & Yellen, G. (2019). Quantitative in vivo imaging of neuronal glucose concentrations with a genetically encoded fluorescence lifetime sensor. Journal of Neuroscience Research, 97(8), 946–960.
Philip, V., Newton, D., Oh, H., Collins, S., Bercik, P., & Sibille, E. (2019). The Effect of Gut Microbiota on Glutamatergic/GABAergic Gene Expression in Adult Mice. Biological Psychiatry, 85, S127–S128.
Bowen, Z., Winkowski, D. E., Seshadri, S., Plenz, D., & Kanold, P. O. (2019). Neuronal avalanches in input and associative layers of auditory cortex. Frontiers in Systems Neuroscience, 13, 45.
Burgold, J., Schulz-Trieglaff, E. K., Voelkl, K., Gutiérrez-Ángel, S., Bader, J. M., Hosp, F., ... & Dudanova, I. (2019). Cortical circuit alterations precede motor impairments in Huntington’s disease mice. Scientific Reports, 9(1), 6634.
Matovic, S., Ichiyama, A., Igarashi, H., Salter, E. W., Wang, X. F., Henry, M., ... & Inoue, W. (2019). Stress-induced neuronal hypertrophy decreases the intrinsic excitability in stress habituation. bioRxiv, 593665.
Weissenberger, Y., King, A. J., & Dahmen, J. C. (2019). Decoding mouse behavior to explain single-trial decisions and their relationship with neural activity. bioRxiv, 567479.
Ceriani, F., Hendry, A., Jeng, J. Y., Johnson, S. L., Stephani, F., Olt, J., ... & Marcotti, W. (2019). Coordinated calcium signalling in cochlear sensory and non-sensory cells refines afferent innervation of outer hair cells. The EMBO Journal, 38(9), e99839.
Lee, K. S., Vandemark, K., Mezey, D., Shultz, N., & Fitzpatrick, D. (2019). Functional synaptic architecture of callosal inputs in mouse primary visual cortex. Neuron, 101(3), 421-428.
2018
Marvin, J. S., Scholl, B., Wilson, D. E., Podgorski, K., Kazemipour, A., Müller, J. A., ... & Looger, L. L. (2018). Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nature Methods, 15(11), 936–939.
Moeyaert, B., Holt, G., Madangopal, R., Perez-Alvarez, A., Fearey, B. C., Trojanowski, N. F., ... & Schreiter, E. R. (2018). Improved methods for marking active neuron populations. Nature Communications, 9(1), 440.
Chen, C. L., Hermans, L., Viswanathan, M. C., Fortun, D., Aymanns, F., Unser, M., ... & Ramdya, P. (2018). Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nature Communications, 9(1), 4390.
Corns, L. F., Johnson, S. L., Roberts, T., Ranatunga, K. M., Hendry, A., Ceriani, F., ... & Marcotti, W. (2018). Mechanotransduction is required for establishing and maintaining mature inner hair cells and regulating efferent innervation. Nature Communications, 9(1), 4015.
Saleem, A. B., Diamanti, E. M., Fournier, J., Harris, K. D., & Carandini, M. (2018). Coherent encoding of subjective spatial position in visual cortex and hippocampus. Nature, 562(7725), 124-127.
Dipoppa, M., Ranson, A., Krumin, M., Pachitariu, M., Carandini, M., & Harris, K. D. (2018). Vision and locomotion shape the interactions between neuron types in mouse visual cortex. Neuron, 98(3), 602-615.
Gillet, S. N., Kato, H. K., Justen, M. A., Lai, M., & Isaacson, J. S. (2018). Fear learning regulates cortical sensory representations by suppressing habituation. Frontiers in Neural Circuits, 11, 112.
2017
Scholl, B., Wilson, D. E., & Fitzpatrick, D. (2017). Local order within global disorder: synaptic architecture of visual space. Neuron, 96(5), 1127-1138.
Klapoetke, N. C., Nern, A., Peek, M. Y., Rogers, E. M., Breads, P., Rubin, G. M., ... & Card, G. M. (2017). Ultra-selective looming detection from radial motion opponency. Nature Neuroscience, 551(7679), 237-241.
Kato, H. K., Asinof, S. K., & Isaacson, J. S. (2017). Network-level control of frequency tuning in auditory cortex. Neuron, 95(2), 412-423.
Lu, R., Sun, W., Liang, Y., Kerlin, A., Bierfeld, J., Seelig, J.D., W... & Ji, N. (2017). Video-rate volumetric functional imaging of the brain at synaptic resolution. Nature Neuroscience, 20(4), 620-628.
2016
Mongeon, R., Venkatachalam, V., & Yellen, G. (2016). Cytosolic NADH-NAD+ redox visualized in brain slices by two-photon fluorescence lifetime biosensor imaging.. Antioxid Redox Signal, 25(10), 553-563.
Pachitariu, M., Stringer, C., Schröder, S., Dipoppa, M., Rossi, L. F., Carandini, M., & Harris, K. D. (2016). Suite2p: beyond 10,000 neurons with standard two-photon microscopy. bioRxiv, 061507.
Rose, T., Jaepel, J., Hübener, M., & Bonhoeffer, T. (2016). Cell-specific restoration of stimulus preference after monocular deprivation in the visual cortex. Science, 352(6291), 1319–1322.
Strobl, M. J., Freeman, D., Patel, J., Poulsen, R., Wendler, C. C., Rivkees, S. A., & Coleman, J. E. (2016). Opposing effects of maternal hypo-and hyperthyroidism on the stability of thalamocortical synapses in the visual cortex of adult offspring.. Cerebral Cortex, 27(5), 3015-3027.
Lee, K. S., Huang, X., & Fitzpatrick, D. (2016). Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature, 533(7601), 90-94.
Monai, H., Ohkura, M., Tanaka, M., Oe, Y., Konno, A., Hirai, H., ... & Hirase, H. (2016). Calcium imaginq reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nature Communications, 7(1), 11100.
Ganmor, E., Krumin, M., Rossi, L. F., Carandini, M., & Simoncelli, E. P. (2016). Direct estimation of firing rates from calcium imaging data. arXiv, 1601.00364.
2015
Roth, M. M., Dahmen, J.C., Muir, D. R., Imhof, F., Martini, F. J., & Hofer, S. B. (2015). Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nature Neuroscience, 19(2), 299-307.
Barnstedt, O., Keating, P., Weissenberger, Y., King, A. J., & Dahmen, J. C. (2015). Functional microarchitecture of the mouse dorsal inferior colliculus revealed through in vivo two-photon calcium imaging.. Journal of Neuroscience, 35(31), 10927-10939.
Chen, S. X., Kim, A. N., Peters, A. J., & Komiyama, T. (2015). Subtype-specific plasticity of inhibitory circuits in motor cortex during motor learning. Nature Neuroscience, 18(8), 1109-1115.
Jia, Y., Zhang, S., Miao, L., Wang, J., Jin, Z., Gu, B., ... & Li, Z. (2015). Activation of platelet protease-activated receptor-1 induces epithelial-mesenchymal transition and chemotaxis of colon cancer cell line SW620. Oncology Reports, 33(6), 2681-2688.
Lu, W., Tang, Y., Zhang, Z., Zhang, X., Yao, Y., Fu, C., ... & Ma, G. (2015). Inhibiting the mobilization of Ly6Chigh monocytes after acute myocardial infarction enhances the efficiency of mesenchymal stromal cell transplantation and curbs myocardial remodeling.. American Journal of Translational Research, 7(3), 587-597.
Boyd, A. M., Kato, H. K., Komiyama, T., & Isaacson, J. S. (2015). Broadcasting of cortical activity to the olfactory bulb. Cell Reports, 10(7), 1032-1039.
Cossell, L., Iacaruso, M. F., Muir, D. R., Houlton, R., Sader, E. N., Ko, H., H... & Mrsic-Flogel, T. D. (2015). Functional organization of excitatory synaptic strength in primary visual cortex. Nature, 518(7539), 399-403.
2014
Partridge, J. G., Lewin, A. E., Yasko, J. R., & Vicini, S. (2014). Contrasting actions of group I metabotropic glutamate receptors in distinct mouse striatal neurones. Journal of Physiology, 592(13), 2721-2733.
Peters, A. J., Chen, S, X., Komiyama, T. (2014). Emergence of reproducible spatiotemporal activity during motor learning. Nature, 510(7504), 263-267.
Ehmke, T., Nitzsche, T. H., Knebl, A., & Heisterkamp, A. (2014). Molecular orientation sensitive second harmonic microscopy by radially and azimuthally polarized light. Biomedical Optics Express, 5(7), 2231-46.
Liu, J., Wu, N., Ma, L., Liu, M., Liu, G., Zhang, Y., & Lin, X. (2014). Oleanolic acid suppresses aerobic glycolysis in cancer cells by switching pyruvate kinase type M isoforms. PLoS One, 9(3), e91606.
Palmer, L. M., Shai, A. S., Reeve, J.E., Anderson, H. L., Paulsen, O., & Larkum, M. E. (2014). NMDA spikes enhance action potential generation during sensory input. Nature Neuroscience, 17(3), 383-390.
Cai, F., Yu, J., Qian, J., Wang, Y., Chen, Z., Huang, J., ... & He, S. (2014). Use of tunable second-harmonic signal from KNbO3 nanoneedles to find optimal wavelength for deep-tissue imaging. Laser & Photonics Reviews, 8(6), 865-874.
2013
Kato, H. K., Gillet, S. N., Peters, A. J., Isaacson, J. S., & Komiyama, T. (2013). Parvalbumin-expressing interneurons linearly control olfactory bulb output. Neuron, 80(5), 1218-1231.
Takata, N., Nagai, T., Ozawa, K., Oe, Y., Mikoshiba, K., & Hirase, H. (2013). Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One, 8(6), e66525.
![]()
The full source code for ThorImage®LS is available for owners of a Bergamo®, Cerna® Confocal, Veneto®, or confocal microscope. Click here to receive your copy.
ThorImage®LS Software
ThorImageLS is an open-source image acquisition program that controls Thorlabs' microscopes, as well as supplementary external hardware. From prepared-slice multiphoton Z-stacks to simultaneous in vivo photoactivation and imaging, ThorImageLS provides an integrated, modular workspace tailored to the individual needs of the scientist. Its workflow-oriented interface supports single image, Z-stacks, time series, and image streaming acquisition, visualization, and analysis. See the video to the lower right for a real-time view of data acquisition and analysis with ThorImageLS.
ThorImageLS is included with a Thorlabs microscope system purchase and is open source, allowing full customization of software features and performance. ThorImageLS also includes Thorlabs’ customer support and regular software updates to continually meet the imaging demands of the scientific community.
For additional details, see the full web presentation.
Advanced Software Functionality
- Multi-Column Customizable Workspace
- Image Acquisition Synced with Hardware Inputs and Timing Events
- Live Image Correction and ROI Analysis
- Independent Galvo-Galvo and Galvo-Resonant Scan Areas and Geometries
- Tiling for High-Resolution Large-Area Imaging
- Independent Primary and Secondary Z-Axis Control for Fast Deep-Tissue Scans
- Automated Image Capture with Scripts
- Compatible with ImageJ Macros
- Multi-User Settings Saved for Shared Workstations
- Individual Colors for Detection Channels Enable Simple Visual Analysis
Seamless Integration with Experiments
- Simultaneous Multi-Point Photoactivation and Imaging with Spatial Light Modulator
- Fast Z Volume Acquisition with PFM450E or Third-Party Objective Scanners
- Electrophysiology Signaling
- Wavelength Switching with Tiberius® Laser or Coherent Chameleon Lasers
- Pockels Cell ROI Masking
- Power Ramped with Depth to Minimize Damage and Maximize Signal-to-Noise
New Functionality: Version 4.3 (Click to Expand for More Details)
Please contact ImagingTechSupport@thorlabs.com to obtain the latest ThorImageLS version compatible with your microscope. Because ThorImageLS 4.x adds significant new features over 3.x, 2.x and 1.x versions, it may not be compatible with older microscopes. We continue to support older software versions for customers with older hardware. See the full web presentation for functionality of previous versions.
- Added Support for the Toptica iChrome CLE-50 Laser
- Added Support for 3P Imaging
- Added Support for the CS126MU, CS165MU, and CC505MU Monochrome Cameras
- Added Support for a Mini-Circuits® Switch Box
- Added Support for PMT3100R (Included in Some Bergamo II Multiphoton Imaging Systems)
- Added Configurable Channel View Layout (Horizontal and Vertical)
- Added Improved Scan Path Realignment and Added Ability to Save Multiple Reference Images for Multiple Targets
- Allows for Simplified Relocation to Same Target Day to Day
- Added New Features for SLM Operation
- Added 3D Mode Toggle
- Added Z Offset
- Added Ability to Export Patterns
- Added Set Zero% and Delete All Buttons
- Added Pattern Center to Display as "0" Point
- Added SLM Control Panel Advanced Mode Enhancement
- Added New Optional Delay Between Epochs
- Added SLM Control Panel Import/Export Enhancement
- Ability to Export Table of SLM Patterns
- Added New SLM Settings
- Added to ThorSLMSettings.xml and Application Settings
- Added Ability to Offset the Z Position of Pattern Points in New 3D Mode
- Enhanced ORCA Fusion Features
- Updated Exposure Calculation for Master Pulse Mode
- Added Option to Enable Water Cooling Control
- Added Improved Performance When Switching Modalities
- Enhanced Two-Way Scanning
- Two-Way Scanning is Now Allowed Up to a Pixel Density of 4096 x 4096 When Only One Channel is Selected
- Only Selectable by Dragging the Slider Bar to the Desired Pixel Density in One-Way Before Switching to Two-Way
- Added Camera Frame Rate Control
- Added UI Control of Two Blue Mini-Circuits® Switch Boxes
- Added 3D SLM
- Added Continuous Preview and Enhanced Orthogonal Views for Z-Stacks
- Added Improved Laser Safety Control for Digital Switches When Switches Are Configured for Laser Switching
- Added Control for Stimulus Shutter Operation When Using Stimulus Capture Mode
- Added Camera Frame Rate Control
- Added a New Section to Control the Frame Rate for CMOS Cameras with this Functionality
- Added Image-Based Autofocus
- Added Ability to Find the Optimal Focus Point of the Sample Based on Image Contrast
- Added Automatic Version Update Checker
- When Connected to Internet a Version Update Check Will Occur as the Splash Screen Loads When ThorImageLS Is Started Up
- Added Signal Generator Analog Mode
- Allows Custom Control of Analog Modulation
- Added New Continuous Button for Repeated Preview
- Allows for Fast Location Sample in XZ and YZ Line Scan Mode
- Added Enhanced IPC Communication
- Added IPC Command to Load an Experiment or a Template
- Added IPC Commands to Move X, Y, Z, and Secondary Z Stages
- Added IPC Commands that Get Sent from ThorImageLS Every Time a File Is Saved During T Series Experiments
Features of ThorImage®LS
| Supported Imaging Platforms | |
|---|---|
 Bergamo® II Multiphoton Microscopes |
 Veneto® Inverted Microscopes |
 Confocal Imaging Systems |
 Hyperspectral Imaging System |
| Laser Scanning | |||
|---|---|---|---|
| Scan Path Wavelength Range | 450 - 1100 nm, 680 - 1300 nm, or 800 - 1800 nm | ||
| Scan Paths | Resonant-Galvo-Galvo Scanner, Galvo-Resonant Scanners, Galvo-Galvo Scanners, or Spatial Light Modulator; Single or Dual Scan Paths |
||
| Scan Speed | 8 kHz Resonant-Galvo-Galvo or Galvo-Resonant |
2 fps at 4096 x 4096 Pixels 30 fps at 512 x 512 Pixels 400 fps at 512 x 32 Pixels |
|
| 12 kHz or Galvo-Resonant |
4.4 fps at 2048 x 2048 Pixels 45 fps at 512 x 512 Pixels 600 fps at 512 x 32 Pixels |
||
| Galvo-Galvo | 3 fps at 512 x 512 Pixels 48 fps at 512 x 32 Pixels 70 fps at 32 x 32 Pixels Pixel Dwell Time: 0.4 to 20 µs |
||
| Galvo-Galvo Scan Modes | Imaging: Line, Polyline, Square, or Rectangle Non-Imaging: Circle, Ellipse, Polygon, or Point |
||
| Field of View | 20 mm Diagonal Square (Max) at the Intermediate Image Plane [12 mm Diagonal Square (Max) for 12 kHz Scanner] |
||
| Scan Zoom | 1X to 16X (Continuously Variable) | ||
| Scan Resolution | Up to 2048 x 2048 Pixels (Bi-Directional) [Up to 1168 x 1168 Pixels for 12 kHz Scanners] Up to 4096 x 4096 Pixels (Unidirectional) [Up to 2336 x 2336 Pixels for 12 kHz Scanners] |
||
| Compatible Objective Threadings | M34 x 1.0, M32 x 0.75, M25 x 0.75, and RMS | ||
| Multiphoton Signal Detection | ||
|---|---|---|
| Epi-Detection | Up to Four Ultrasensitive GaAsP PMTs, Cooled or Non-Cooled | |
| Forward-Direction Detection | Two Ultrasensitive GaAsP PMTs | |
| Maximum of Four PMTs Controlled by the Software at a Given Time | ||
| Collection Optics | 8°, 10°, or 14° Collection Angle (Angles Quoted When Using an Objective with a 20 mm Entrance Pupil) Easy-to-Exchange Emission Filters and Dichroic Mirrors |
|
| Confocal Imaging | ||
|---|---|---|
| Motorized Pinhole Wheel with 16 Round Pinholes from Ø25 µm to Ø2 mm Two to Four Laser Lines (488 nm Standard; Other Options Range from 405 nm to 660 nm) Standard Multialkali or High-Sensitivity GaAsP PMTs Easy-to-Exchange Emission Filters and Dichroic Mirrors |
| Widefield Viewing | ||
|---|---|---|
| Manual or Motorized Switching Between Scanning and Widefield Modes Illumination Provided via LED or Liquid Light Guide C-Mount Threads for Scientific Cameras |
| Transmitted Light Imaging | ||
|---|---|---|
| Differential Interference Contrast (DIC) or Dodt Gradient Contrast Widefield or Laser Scanned Illumination Provided by Visible and/or NIR LEDs Compatible with Air or Oil Immersion Condensers |
| Three-Photon Imaging | ||
|---|---|---|
| Scan Optics for 800 - 1800 nm Range Achieve Reduced Background Scatter for Greater Sensitivity in Deep Tissue Imaging |
| Volume Imaging Using Bessel Beams | ||
|---|---|---|
| 3D Volumetric Functional Imaging at Video Frame Rates Enhanced Temporal Resolution for Studying Internal Systems at Cellular Lateral Resolution In Vivo |
| Translation | ||||
|---|---|---|---|---|
| Microscope Body Rotation (Rotating Bodies Only) |
0° to 90° or -45° to +45° Around Objective Focus 0.1° Encoder Resolution |
|||
| Coarse Elevator Base Z (Rotating Bodies Only) |
5" (127 mm) Total Travel; 1 µm Encoder Resolution | |||
| Fine Microscope Body X and Y | 2" (50.8 mm) Total Travel; 0.5 µm Encoder Resolution | |||
| Fine Microscope Arm Z | 1" (25.4 mm) Total Travel; 0.1 µm Encoder Resolution | |||
| Fine Objective Z (Piezo Objective Scanner) |
Open Loop: 600 µm ± 10% Travel Range; 1 nm Resolution Closed Loop: 450 µm Travel Range; 3 nm Resolution |
|||
Thorlabs recognizes that each imaging application has unique requirements.
If you have any feedback, questions, or need a quotation, please use our
multiphoton microscopy contact form or call (703) 651-1700.

Click to Enlarge
China Demo Room
Try Our Microscopes In Person or Virtually
Thorlabs' sales engineers and field service staff are based out of nine offices across four continents. We look forward to helping you determine the best imaging system to meet your specific experimental needs. Our customers are attempting to solve biology's most important problems; these endeavors require matching systems that drive industry standards for ease of use, reliability, and raw capability.
Thorlabs' worldwide network allows us to operate demo rooms in a number of locations where you can see our systems in action. We welcome the opportunity to work with you in person or virtually. A demo can be scheduled at any of our showrooms or virtually by contacting ImagingSales@thorlabs.com.
Customer Support Sites
(Click Each Location for More Details)
Newton, New Jersey, USA
Thorlabs Headquarters
43 Sparta Avenue
Newton, NJ 07860
Customer Support
- Phone: (973) 300-3000
- E-mail: techsupport@thorlabs.com
Ely, United Kingdom
Thorlabs Ltd.
1 Saint Thomas Place, Ely
Ely CB7 4EX
Customer Support
- Phone: +44 (0)1353-654440
- E-mail: techsupport.uk@thorlabs.com
Bergkirchen, Germany
Thorlabs GmbH
Münchner Weg 1
85232 Bergkirchen
Customer Support
- Phone: +49 (0) 8131-5956-0
- E-mail: europe@thorlabs.com
Le Mesnil-le-Roi, France
Thorlabs SAS
14 rue Gambetta
78600 Le Mesnil-le-Roi
Customer Support
- Phone: +33 (0) 970 440 844
- E-mail: techsupport.fr@thorlabs.com
São Carlos, SP, Brazil
Thorlabs Vendas de Fotônicos Ltda.
Rua Rosalino Bellini, 175
Jardim Santa Paula
São Carlos, SP, 13564-050
Customer Support
- Phone: +55-16-3413 7062
- E-mail: brasil@thorlabs.com
Demo Rooms and Customer Support Sites
(Click Each Location for More Details)
Sterling, Virginia, USA
Thorlabs Imaging Systems HQ
108 Powers Court
Sterling, VA 20166
Customer Support
- Phone: (703) 651-1700
- E-mail: ImagingTechSupport@thorlabs.com
Demo Rooms
- Bergamo® II Series Multiphoton Microscopes
- Veneto® Inverted Microscopes
- Four-Channel Cerna®-Based Confocal Microscopes
- Cerna Hyperspectral Imaging System
- Cerna Birefringence Imaging Microscopes
- Multiphoton Mesoscope
- OCT Systems: Telesto® and Ganymede™
Lübeck, Germany
Thorlabs GmbH
Maria-Goeppert-Straße 9
23562 Lübeck
Customer Support
- Phone: +49 (0) 8131-5956-40840
- Email: oct@thorlabs.com
Demo Rooms
- Ganymede™ Series SD-OCT Systems
- Telesto® Series SD-OCT Systems
- Telesto® Series PS-OCT Systems
- Atria® Series SS-OCT Systems
- Vega™ Series SS-OCT Systems
Nerima-ku, Tokyo, Japan
Thorlabs Japan, Inc.
3-6-3 Kitamachi
Nerima-ku, Tokyo 179-0081
Customer Support
- Phone: +81-3-6915-7701
- Email: sales@thorlabs.jp
Demo Rooms
- Four-Channel Cerna®-Based Confocal Systems
- Cerna® Modular Brightfield Microscopes
- OCT Systems: Ganymede™
Shanghai, China
Thorlabs China
Room A101, No. 100, Lane 2891, South Qilianshan Road
Shanghai 200331
Customer Support
- Phone: +86 (0)21-60561122
- Email: techsupport-cn@thorlabs.com
Demo Rooms
- Bergamo® II Series Multiphoton Microscopes
- Cerna Birefringence Imaging Microscopes
- OCT Systems: Telesto® and Ganymede™
| Posted Comments: | |
gaiqing Wang
(posted 2020-05-07 11:13:33.577) I am looking for a cheap way to do confocal imaging in vivo. Is this Bergamo II Series Multiphoton Microscope my best option? Can you send me a quote? YLohia
(posted 2020-05-07 09:45:11.0) Thank you for contacting Thorlabs. We will reach out to you directly to discuss your requirements. jfpena
(posted 2016-12-19 18:15:55.003) I am looking for a cheap way to do confocal imaging in vivo. Is this Bergamo II Series Multiphoton Microscope my best option? Can you send me a quote? tfrisch
(posted 2016-12-22 11:44:31.0) Hello, thank you for contacting Thorlabs. A member of our Imaging Team will reach out to you directly to discuss this system and your application. birech
(posted 2016-11-17 06:33:49.463) I asked for a price quote for this product, Bergamo II Series Multiphoton Microscopes three days ago. I am working at the University of Nairobi in Kenya and would wish to order one.
Regards,
Birech tfrisch
(posted 2016-11-17 06:56:23.0) Hello, thank you for contacting Thorlabs. I have forwarded this request to our Imaging Sales Team. I apologize for the delay. |
 Products Home
Products Home










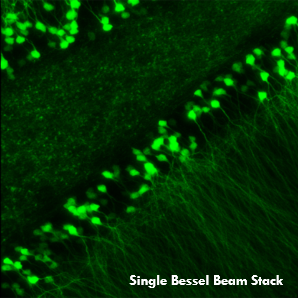
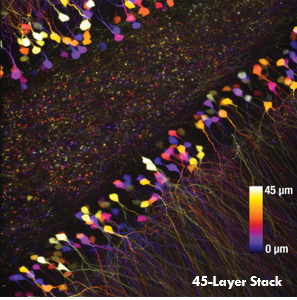
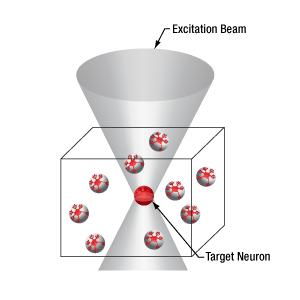





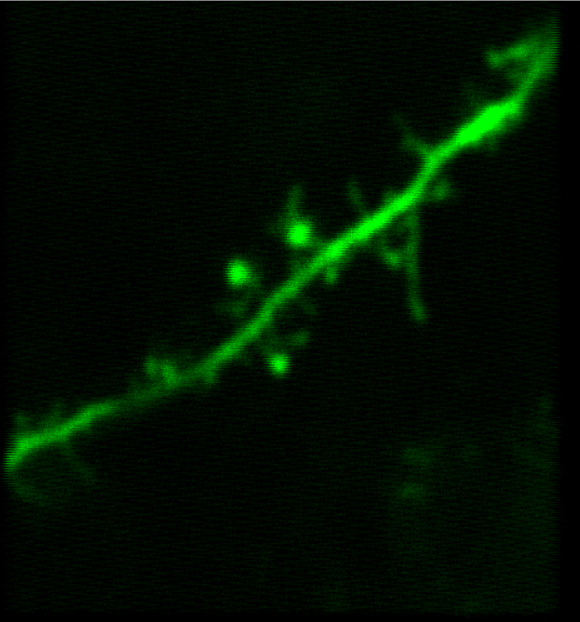
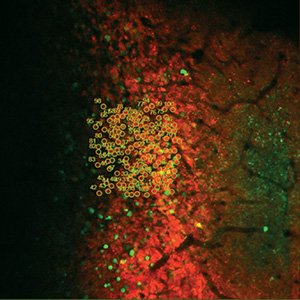

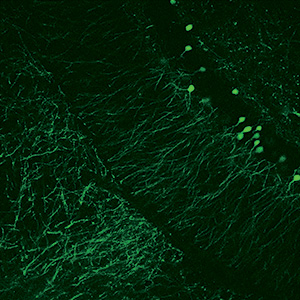

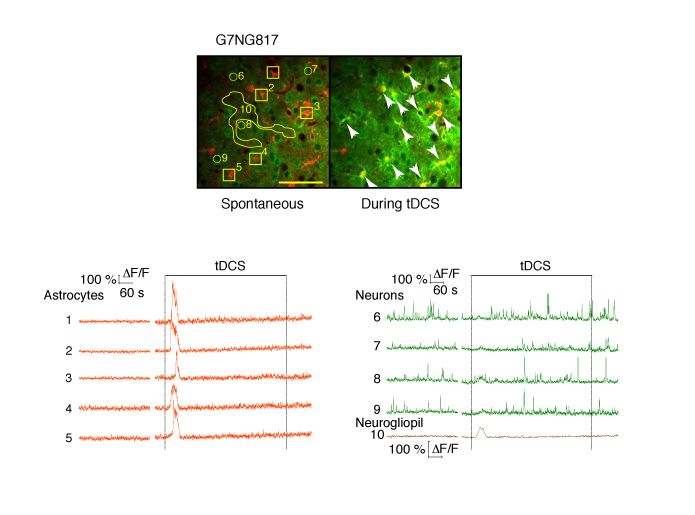

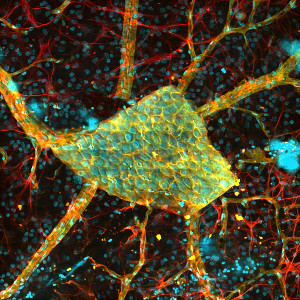






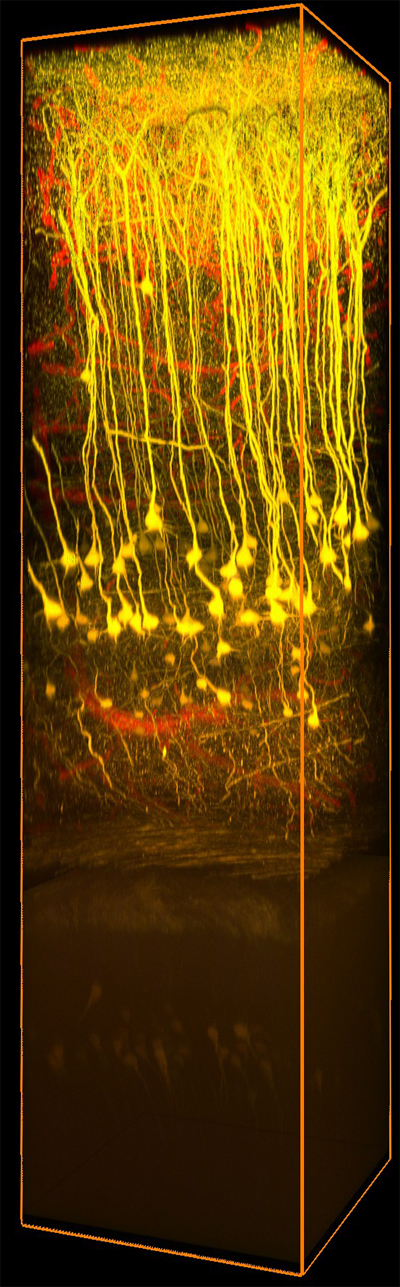
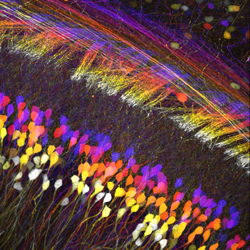
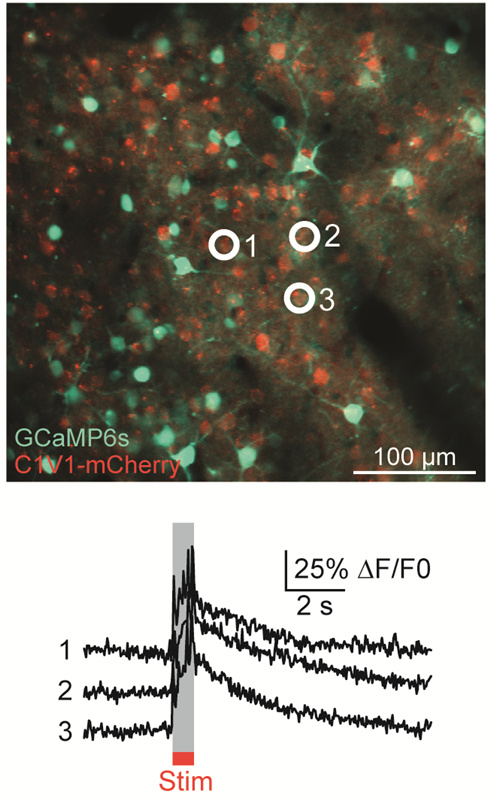
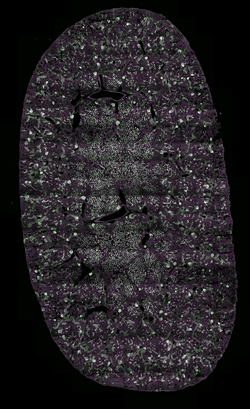
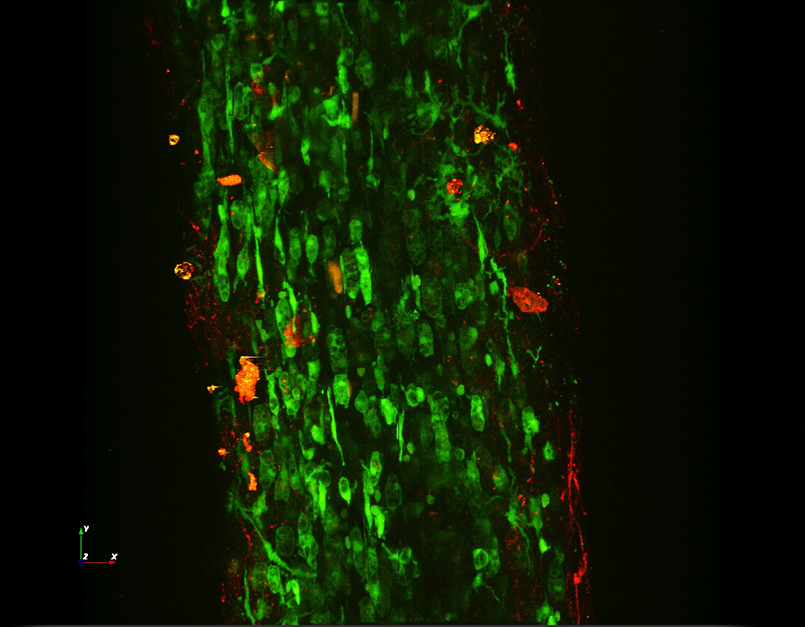
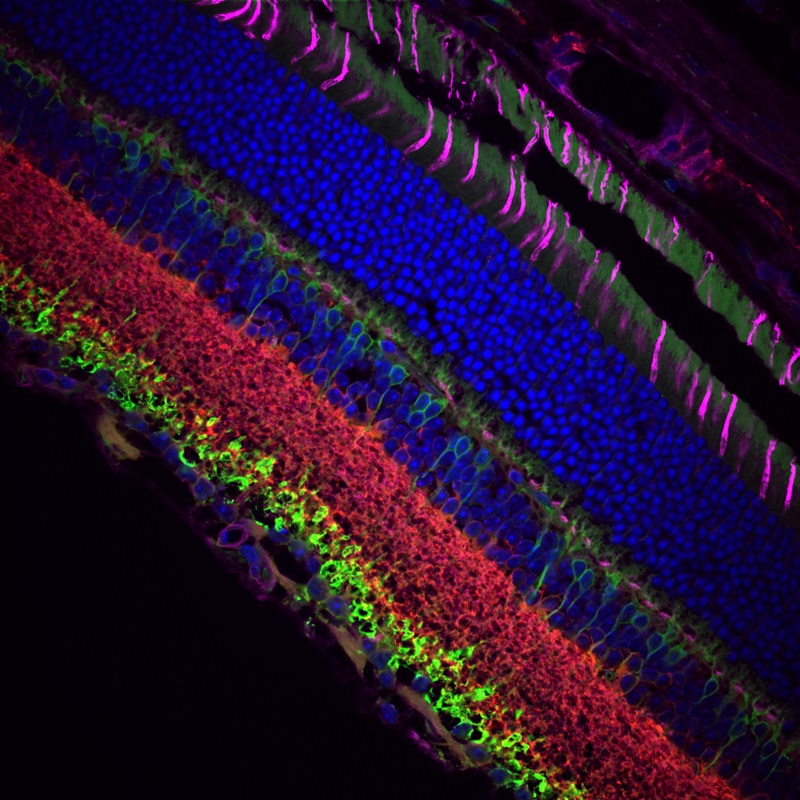
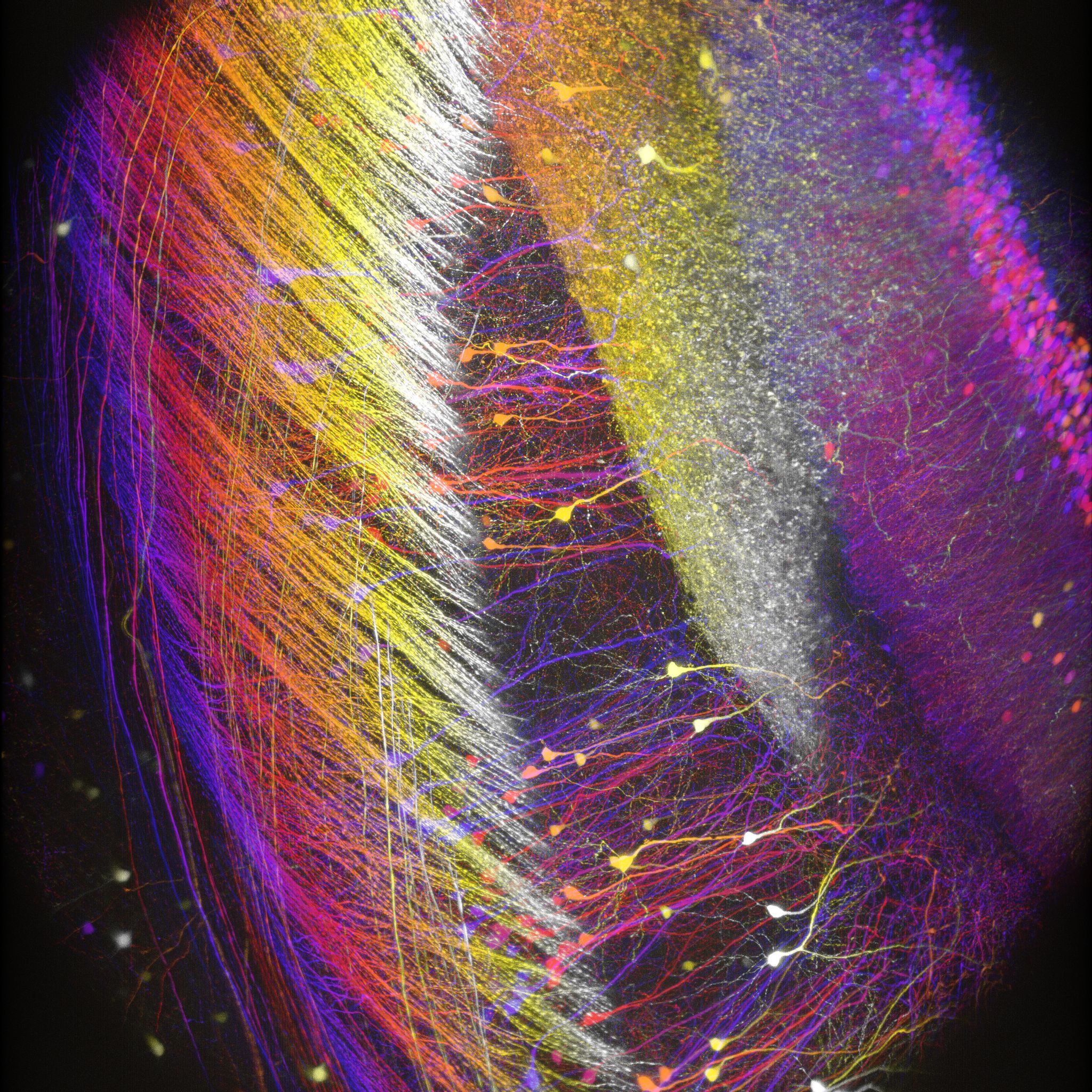
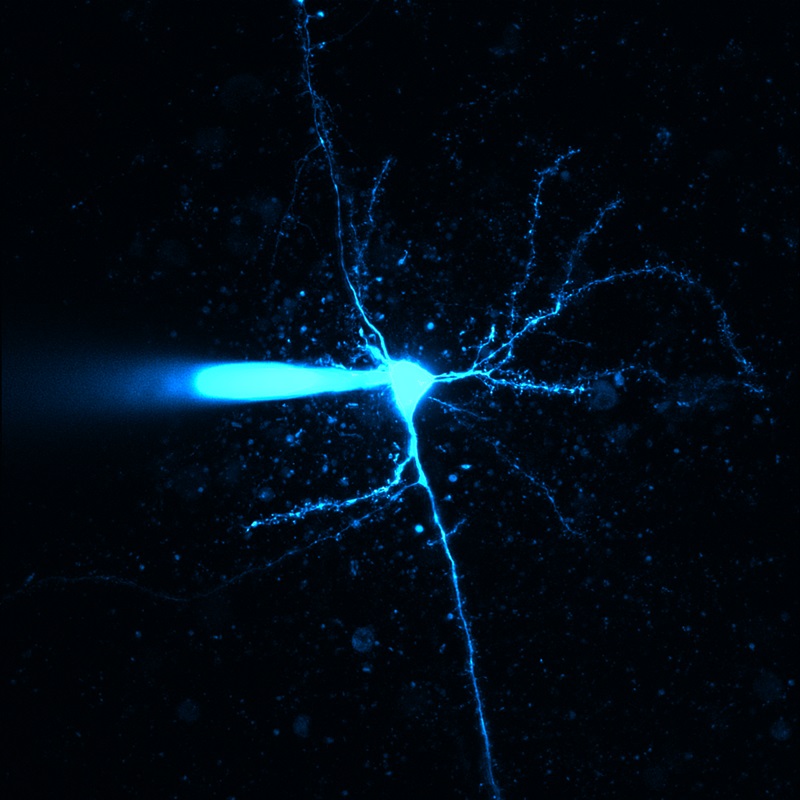
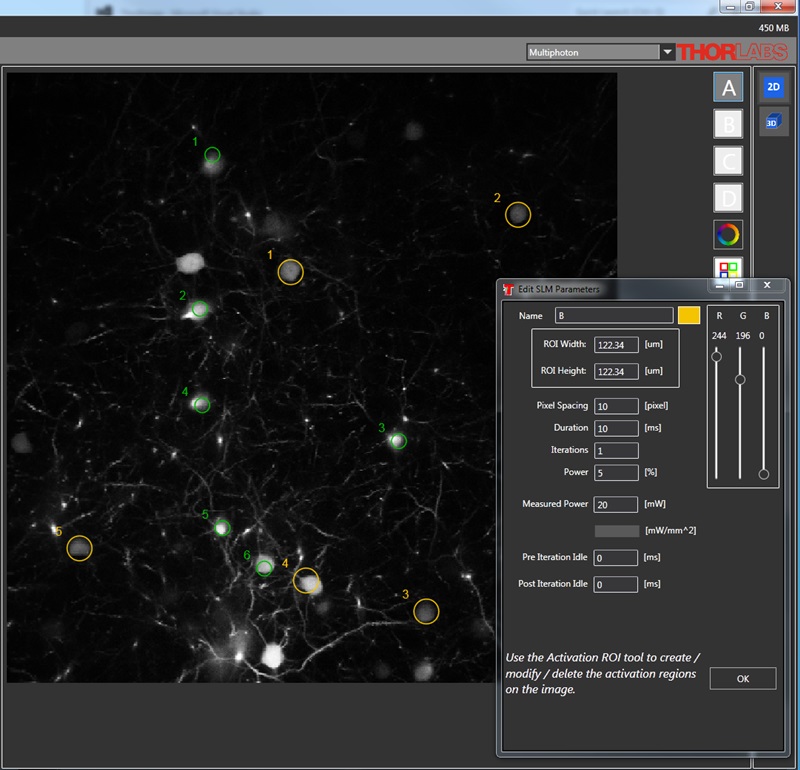
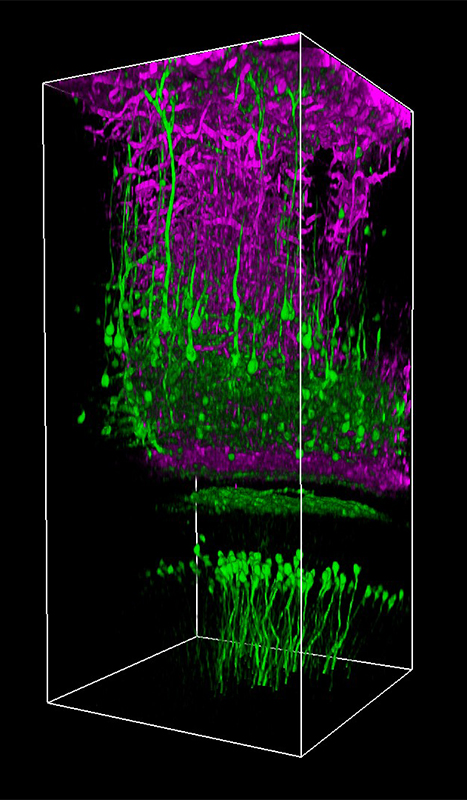
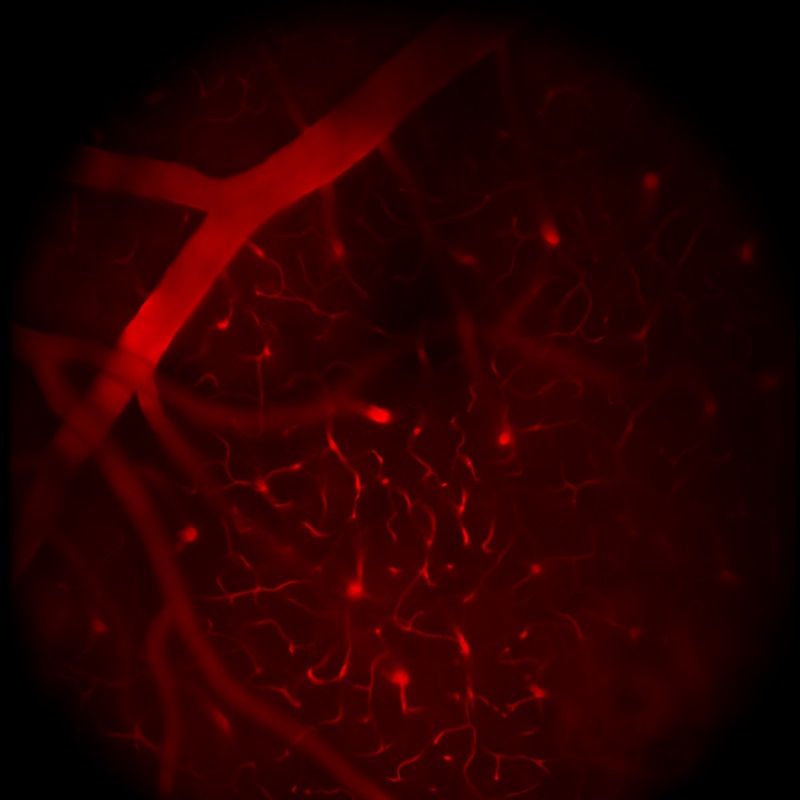
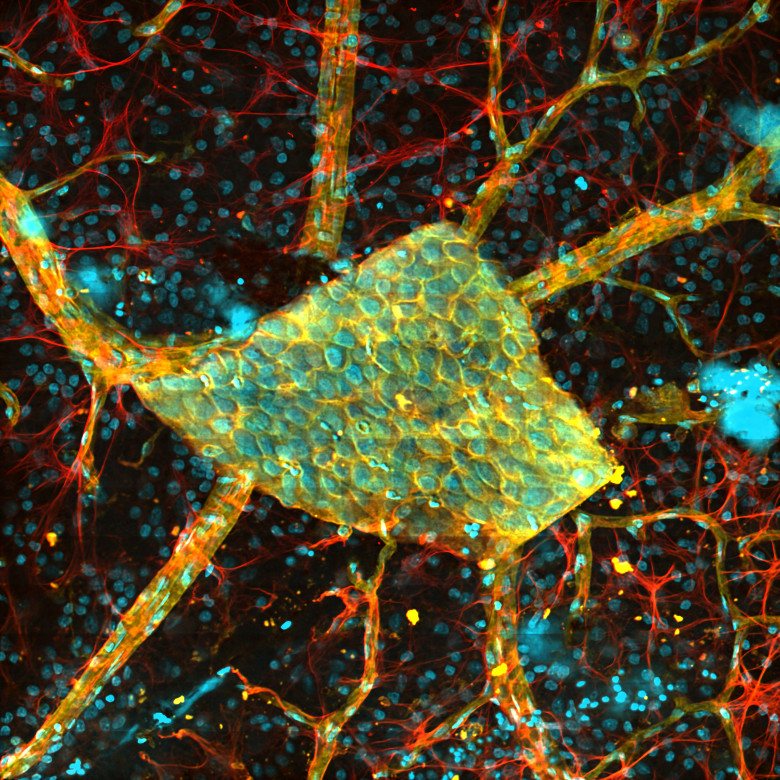
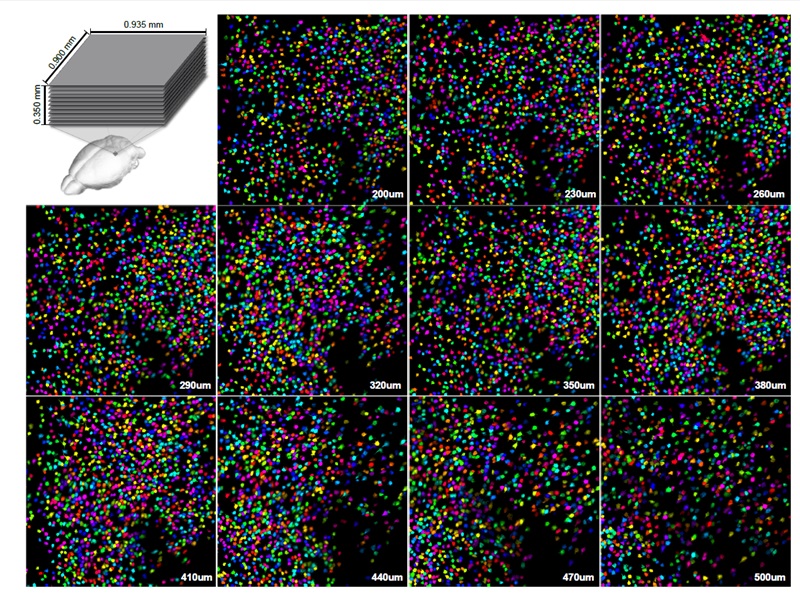
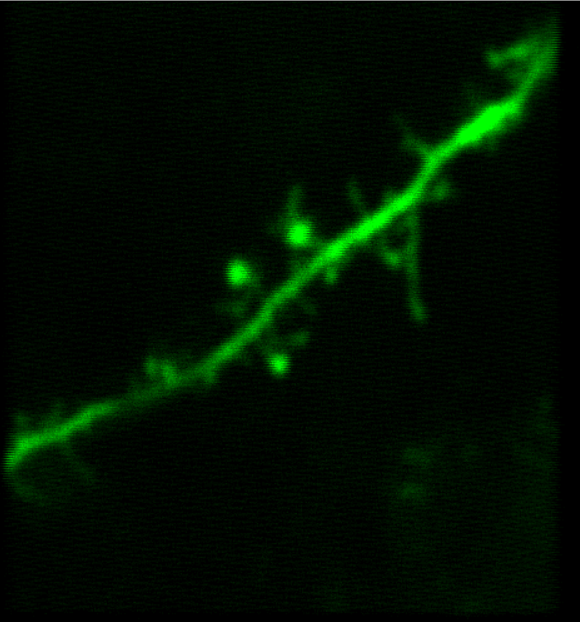
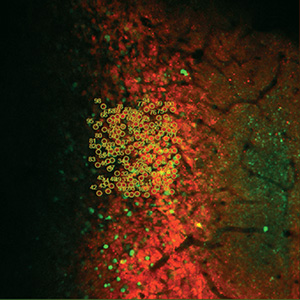
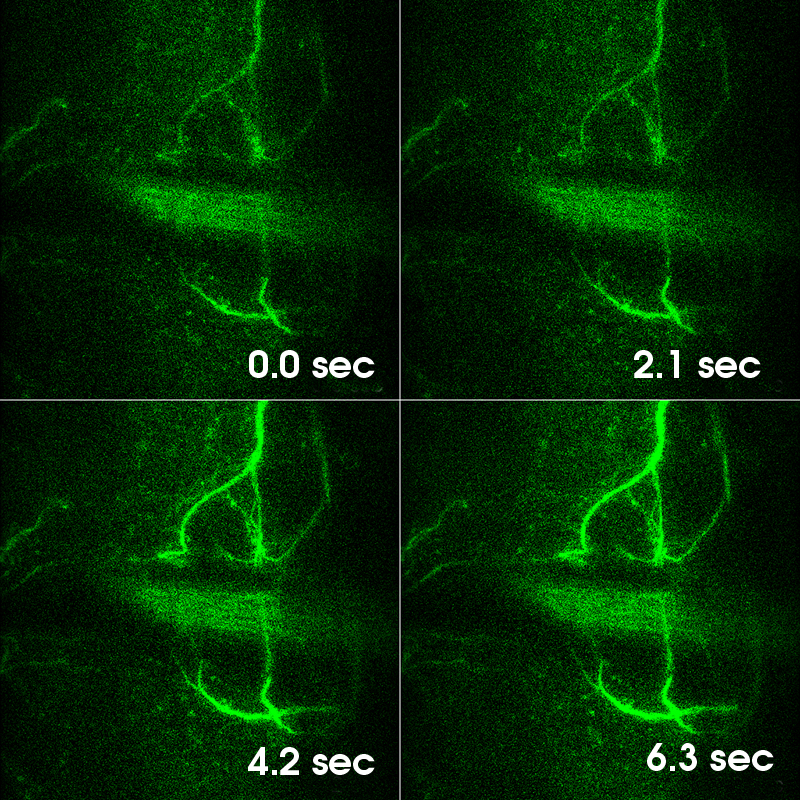














 Multiphoton Microscopes: Bergamo® II Series
Multiphoton Microscopes: Bergamo® II Series